Image-Based Profiling of Patient-Derived Pancreatic Tumor-Stromal Cell Interactions Within a Micropatterned Tumor Model
- PMID: 30348057
- PMCID: PMC6201185
- DOI: 10.1177/1533033818803632
Image-Based Profiling of Patient-Derived Pancreatic Tumor-Stromal Cell Interactions Within a Micropatterned Tumor Model
Abstract
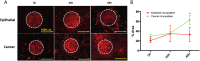
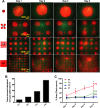
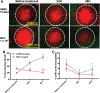
Pancreatic cancer is one of the most aggressive cancers with a 5-year patient survival rate of 8.2% and limited availability of therapeutic agents to target metastatic disease. Pancreatic cancer is characterized by a dense stromal cell population with unknown contribution to the progression or suppression of tumor growth. In this study, we describe a microengineered tumor stromal assay of patient-derived pancreatic cancer cells to study the heterotypic interactions of patient pancreatic cancer cells with different types of stromal fibroblasts under basal and drug-treated conditions. The population dynamics of tumor cells in terms of migration and viability were visualized as a functional end point. Coculture with cancer-associated fibroblasts increased the migration of cancer cells when compared to dermal fibroblasts. Finally, we imaged the response of a bromodomain and extraterminal inhibitor on the viability of pancreatic cancer clusters surrounding by stroma in microengineered tumor stromal assay. We visualized a codynamic reduction in both cancer and stromal cells with bromodomain and extraterminal treatment compared to the dimethyl sulfoxide-treated group. This study demonstrates the ability to engineer tumor-stromal assays with patient-derived cells, study the role of diverse types of stromal cells on cancer progression, and precisely visualize a coculture during the screening of therapeutic compounds.
Keywords: BET; microengineered tumor stromal assay (µTSA); pancreatic cancer; stroma; tumor microenvironment.
Conflict of interest statement
Figures


Similar articles
-
Fabrication Method of a High-Density Co-Culture Tumor-Stroma Platform to Study Cancer Progression.Methods Mol Biol. 2021;2258:241-255. doi: 10.1007/978-1-0716-1174-6_16. Methods Mol Biol. 2021. PMID: 33340365
-
Unique metabolic features of pancreatic cancer stroma: relevance to the tumor compartment, prognosis, and invasive potential.Oncotarget. 2016 Nov 29;7(48):78396-78411. doi: 10.18632/oncotarget.11893. Oncotarget. 2016. PMID: 27623078 Free PMC article.
-
Blocking CTGF-Mediated Tumor-Stroma Interplay in Pancreatic Cancer.Trends Mol Med. 2020 Dec;26(12):1064-1067. doi: 10.1016/j.molmed.2020.08.005. Epub 2020 Sep 11. Trends Mol Med. 2020. PMID: 32928643
-
Reshaping the Tumor Stroma for Treatment of Pancreatic Cancer.Gastroenterology. 2018 Mar;154(4):820-838. doi: 10.1053/j.gastro.2017.11.280. Epub 2017 Dec 26. Gastroenterology. 2018. PMID: 29287624 Review.
-
Imaging the microenvironment of pancreatic cancer patient-derived orthotopic xenografts (PDOX) growing in transgenic nude mice expressing GFP, RFP, or CFP.Cancer Lett. 2016 Sep 28;380(1):349-55. doi: 10.1016/j.canlet.2015.12.021. Epub 2015 Dec 29. Cancer Lett. 2016. PMID: 26742463 Review.
References
-
- Hanahan D, Weinberg Robert A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

