Phenotypic and transcriptomic characterization of a wheat tall mutant carrying an induced mutation in the C-terminal PFYRE motif of RHT-B1b
- PMID: 30348083
- PMCID: PMC6196432
- DOI: 10.1186/s12870-018-1465-4
Phenotypic and transcriptomic characterization of a wheat tall mutant carrying an induced mutation in the C-terminal PFYRE motif of RHT-B1b
Abstract
Background: As central regulators of the gibberellic acid (GA) signaling pathway in plants, DELLA proteins function as growth repressors and affect diverse biological processes. The wheat RHT-B1b and RHT-D1b semi-dwarfing alleles, which encode GA-insensitive DELLA proteins, have been widely adopted in modern wheat varieties to improve lodging tolerance and harvest index. However, the molecular mechanisms by which DELLA modulates these responses in wheat remain largely unknown.
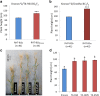
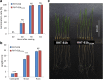
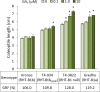
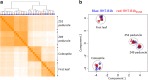
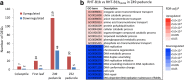
Results: We identified a tall tetraploid wheat mutant line carrying an induced missense mutation (E529K) in the PFYRE motif of RHT-B1b that partially suppressed the semi-dwarf phenotype. The height-increasing effect of RHT-B1bE529K relative to RHT-B1b (19 cm or 21% increase) was significantly smaller than the effect of RHT-B1a (33 cm or 34% increase) relative to RHT-B1b in the same field experiment. The RHT-B1bE529K mutation was also associated with length increases in coleoptiles, seedling shoots, and stem internodes relative to the RHT-B1b allele. We detected no significant differences in germination rate, seedling root length, tiller number, flag leaf size, spike length, or yield components. Using RNA-seq, we compared gene expression profiles of plants encoding RHT-B1b and RHT-B1bE529K in coleoptile, first leaf, and elongating peduncles. We detected limited overlap among tissues of the genes differentially regulated by the two genotypes, and more genes upregulated (77%) than downregulated (23%) in RHT-B1bE529K relative to RHT-B1b. These results suggest that the wheat DELLA protein affects the transcriptome in a tissue-specific manner and that the mutation mainly eliminates or reduces repression functions of the RHT-B1 protein. Our study identified distinct sets of potential DELLA direct or indirect target genes involved in cell wall and carbohydrate metabolisms, cell cycle/division, and hormone pathways.
Conclusions: We identified the hypomorphic RHT-B1bE529K allele that confers an intermediate plant height and coleoptile elongation. This allele can be useful in rain-fed wheat breeding programs where the strong reduction in height and biomass associated with RHT-B1b has detrimental effects. Transcriptomic characterization of different tissues from the plants encoding RHT-B1bE529K and RHT-B1b provided valuable information for identifying DELLA downstream GA response genes in wheat.
Keywords: DELLA; Plant height; RHT1; RNA-seq; Transcriptome; Wheat.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Molecular characterization of Rht-1 dwarfing genes in hexaploid wheat.Plant Physiol. 2011 Dec;157(4):1820-31. doi: 10.1104/pp.111.183657. Epub 2011 Oct 19. Plant Physiol. 2011. PMID: 22013218 Free PMC article.
-
Differential coupling of gibberellin responses by Rht-B1c suppressor alleles and Rht-B1b in wheat highlights a unique role for the DELLA N-terminus in dormancy.J Exp Bot. 2017 Jan 1;68(3):443-455. doi: 10.1093/jxb/erw471. J Exp Bot. 2017. PMID: 28073950 Free PMC article.
-
GA-responsive dwarfing gene Rht12 affects the developmental and agronomic traits in common bread wheat.PLoS One. 2013 Apr 26;8(4):e62285. doi: 10.1371/journal.pone.0062285. Print 2013. PLoS One. 2013. PMID: 23658622 Free PMC article.
-
The genes of the Green Revolution.Trends Genet. 2003 Jan;19(1):5-9. doi: 10.1016/s0168-9525(02)00009-4. Trends Genet. 2003. PMID: 12493241 Review.
-
The knockout of Gγ subunit HvGS3 by CRISPR/Cas9 gene editing improves the lodging resistance of barley through dwarfing and stem strengthening.Theor Appl Genet. 2025 Feb 27;138(3):61. doi: 10.1007/s00122-025-04853-8. Theor Appl Genet. 2025. PMID: 40014102 Review.
Cited by
-
A polyclonal antibody against a recombinantly expressed Triticum aestivum RHT-D1A protein.J Genet Eng Biotechnol. 2020 Sep 16;18(1):52. doi: 10.1186/s43141-020-00072-4. J Genet Eng Biotechnol. 2020. PMID: 32936364 Free PMC article.
-
Lipopolysaccharide and flagellin of Azospirillum brasilense Sp7 influence callus morphogenesis and plant regeneration in wheat.World J Microbiol Biotechnol. 2022 Feb 24;38(4):62. doi: 10.1007/s11274-022-03247-y. World J Microbiol Biotechnol. 2022. PMID: 35199239
-
Harnessing hormone gibberellin knowledge for plant height regulation.Plant Cell Rep. 2022 Oct;41(10):1945-1953. doi: 10.1007/s00299-022-02904-8. Epub 2022 Jul 20. Plant Cell Rep. 2022. PMID: 35857075 Review.
-
Manipulating Gibberellin Control Over Growth and Fertility as a Possible Target for Managing Wild Radish Weed Populations in Cropping Systems.Front Plant Sci. 2020 Mar 19;11:190. doi: 10.3389/fpls.2020.00190. eCollection 2020. Front Plant Sci. 2020. PMID: 32265944 Free PMC article.
References
-
- de Lucas Miguel, Davière Jean-Michel, Rodríguez-Falcón Mariana, Pontin Mariela, Iglesias-Pedraz Juan Manuel, Lorrain Séverine, Fankhauser Christian, Blázquez Miguel Angel, Titarenko Elena, Prat Salomé. A molecular framework for light and gibberellin control of cell elongation. Nature. 2008;451(7177):480–484. doi: 10.1038/nature06520. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

