Recombinant PAPP-A resistant insulin-like growth factor binding protein 4 (dBP4) inhibits angiogenesis and metastasis in a murine model of breast cancer
- PMID: 30348128
- PMCID: PMC6196427
- DOI: 10.1186/s12885-018-4950-0
Recombinant PAPP-A resistant insulin-like growth factor binding protein 4 (dBP4) inhibits angiogenesis and metastasis in a murine model of breast cancer
Abstract
Background: The Insulin-like growth factor (IGF) pathway plays a role in tumour development and progression. In vivo, IGF1 activity is regulated by the IGF binding proteins (IGFBPs). IGFBP4 inhibits the activity of IGF1 but proteolytic cleavage by pregnancy-associated plasma protein-A (PAPP-A) releases active IGF1. A modified IGFBP4, dBP4, which was resistant to PAPP-A cleavage but retained IGF1 binding capacity, was engineered, expressed in Human Embryonic Kidney (HEK) 293 cells and purified. This study examined the effects of dBP4 on IGF1-induced cell migration, invasion and angiogenesis in vitro. The effect of intra-tumour injections of dBP4 on tumour angiogenesis and metastasis was examined using the 4T1.2luc orthotopic model of breast cancer.
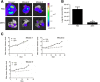
Methods: PAPP-A resistance and IGF binding capacity of dBP4 were characterized by Western blot and surface plasmon resonance, respectively. 4T1.2luc are mouse mammary adenocarcinoma cells transfected with luciferase to allow in vivo imaging. The effect of dBP4 on IGF1-induced Akt activation in 4T1.2luc cells was assessed by Western blot. Cell migration and invasion assays were performed using 4T1.2luc cells. Angiokit™ assays and Matrigel® implants were used to assess the effects of dBP4 on angiogenesis in vitro and in vivo, respectively. An orthotopic breast cancer model - 4T1.2luc cells implanted in the mammary fat pad of BALB/c mice - was used to assess the effect of intra tumour injection of purified dBP4 on tumour angiogenesis and metastasis. Tumour growth and lung metastasis were examined by in vivo imaging and tumour angiogenesis was evaluated by CD31 immunohistochemistry.
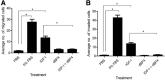
Results: Our engineered, PAPP-A resistant IGFBP4 (dBP4) retained IGF1 binding capacity and inhibited IGF1 activation of Akt as well as IGF1-induced migration and invasion by 4T1.2 mammary adenocarcinoma cells. dBP4 inhibited IGF1-induced angiogenesis in vitro and in Matrigel implants in vivo. Direct intra-tumour injection of soluble dBP4 reduced angiogenesis in 4T1.2 luc mammary tumours tumour and reduced lung metastasis.
Conclusion: A PAPP-A resistant IGFBP4, dBP4, inhibits angiogenesis and metastasis in 4T1.2 mammary fat pad tumours. This study highlights the therapeutic potential of dBP4 as an approach to block the tumour-promoting actions of IGF1.
Keywords: Angiogenesis; IGFBP4/dBP4; Insulin-like growth factor (IGF); Pregnancy associated plasma protein A/PAPP-A.
Conflict of interest statement
Ethics approval
Animal studies were approved by the Ethics Committee of Royal College of Surgeons in Ireland, carried out under animal license guidelines of the Department of Health, Ireland and in accordance with the UK Co-ordinating Committee on Cancer Research (UKCCCR) Guidelines for the Welfare of Animals in Experimental Neoplasia.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Hermanto U, Zong CS, Wang LH. Inhibition of mitogen-activated protein kinase kinase selectively inhibits cell proliferation in human breast cancer cells displaying enhanced insulin-like growth factor I-mediated mitogen-activated protein kinase activation. Cell Growth Differ. 2000;11:655–664. - PubMed
-
- Grey A, Chen Q, Xu X, Callon K, Cornish J. Parallel phosphatidylinositol-3 kinase and p42/44 mitogen-activated protein kinase signaling pathways subserve the mitogenic and antiapoptotic actions of insulin-like growth factor I in osteoblastic cells. Endocrinology. 2003;144:4886–4893. doi: 10.1210/en.2003-0350. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

