Identification of sesquiterpene synthases from the Basidiomycota Coniophora puteana for the efficient and highly selective β-copaene and cubebol production in E. coli
- PMID: 30348159
- PMCID: PMC6198442
- DOI: 10.1186/s12934-018-1010-z
Identification of sesquiterpene synthases from the Basidiomycota Coniophora puteana for the efficient and highly selective β-copaene and cubebol production in E. coli
Abstract
Background: Terpenes are an important and extremely versatile class of secondary metabolites that are commercially used in the pharmaceutical, food and cosmetics sectors. Genome mining of different fungal collections has revealed the genetic basis for a steadily increasing number of putative terpene synthases without any detailed knowledge about their biochemical properties. The analysis and research of this rich genetic source provides a precious basis for the advancing biotechnological production of an almost endless number of valuable natural metabolites.
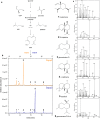
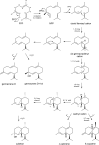
Results: Three annotated terpene synthases from the little investigated Basidiomycota Coniophora puteana were studied in this work. For biochemical characterization, the heterologous expression in E. coli was conducted leading to the identification of two sesquiterpene synthases capable of the highly selective generation of β-copaene and cubebol. These compounds are commercially used as food and flavor additives. The new enzymes show the highest reported product selectivity for their main compounds and therefore represent the first exclusive synthases for β-copaene (62% product selectivity) and cubebol (75% product selectivity) generation. In combination with an optimized heterologous microbial production system, we obtained product titers of 215 mg/L β-copaene and 497 mg/L cubebol.
Conclusion: The reported product selectivity and our generated terpene titers exceed all published biotechnological data regarding the production of β-copaene and cubebol. This represents a promising and economic alternative to extraction from natural plant sources and the associated complex product purification.
Keywords: Basidiomycota; Coniophora puteana; Copaene; Cubebol; Fermentation; Heterologous expression; Phylogenetic analysis; Sesquiterpene.
Figures




References
-
- Hassan SB, Gali-Muhtasib H, Göransson H, Larsson R. Alpha terpineol: a potential anticancer agent which acts through suppressing NF-kappaB signalling. Anticancer Res. 2010;30:1911–1919. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

