Community-based intervention for management of diabetes in Nepal (COBIN-D trial): study protocol for a cluster-randomized controlled trial
- PMID: 30348188
- PMCID: PMC6196417
- DOI: 10.1186/s13063-018-2954-3
Community-based intervention for management of diabetes in Nepal (COBIN-D trial): study protocol for a cluster-randomized controlled trial
Abstract
Background: Type 2 diabetes is one of the fastest emerging chronic diseases in low- and middle-income countries. Population-based approaches, such as involvement of lay health workers offering culturally appropriate diabetes health promotion, may be the blueprint for the management of type 2 diabetes. This study aims to examine the effectiveness of a family-based home health education intervention on type 2 diabetes provided by female community health volunteers (FCHVs) in a semi-urban area of Lekhnath Municipality of Nepal.
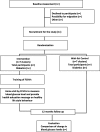
Methods: The COmmunity-Based INtervention for management of Diabetes in Nepal (COBIN-D) trial is a community-based, open-label, two-armed, cluster-randomized trial with seven randomly selected intervention and seven wait-list control clusters. A total of 112 subjects with type 2 diabetes will be recruited from the intervention clusters and 112 subjects from the wait-list control clusters. Based on the Health Belief Model and Social Support Theory, a 12-month family-based lifestyle intervention will be administered through FCHVs. Wait-list control clusters will continue to manage their glycemic condition as usual and their intervention will be delayed for 12 months. Participants will be measured at the beginning of the study and 12 months later. The primary outcome measure of the study will be difference in mean change (from baseline to 1 year) in fasting blood glucose between the two study arms. Impacts will be estimated using intention-to-treat analysis.
Discussion: The COBIN-D is the first study investigating the effect of family-based home health education and screening on blood sugar levels in adults by FCHVs at community level in Nepal. The perspective of this study is to develop and implement, in collaboration with the community, a community-based, culturally sensitive diabetes prevention and control program. It is anticipated that the study can act as a feasible and affordable tool for evidence-based integrated care for improvement of diabetes management and outcomes in Nepal as well as in other low- and middle-income countries.
Trial registration: ClinicalTrials.gov, Identifier: NCT03304158 . Registered retrospectively on 03 October 2017.
Keywords: Community health worker; Community-based; Female community health volunteers; Nepal; Non-communicable diseases; Primary healthcare; Type 2 diabetes.
Conflict of interest statement
Ethics approval and consent to participate
This trial has received ethical approval from the Institutional Review Board at the Nepal Health Research Council, Nepal (Reg.no. 263/2016) on November 10, 2016. Written informed consent will be obtained from study participants and FCHVs prior to enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- World Health Organization . Global Report on Diabetes. Geneva: World Health Organization; 2016.
-
- World Health Organization . Diabetes Mellitus: Fact Sheets. Geneva: World Health Organization; 2015.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical