Multicentre, double-blind, crossover trial to identify the Optimal Pathway for TreatIng neurOpathic paiN in Diabetes Mellitus (OPTION-DM): study protocol for a randomised controlled trial
- PMID: 30348206
- PMCID: PMC6196556
- DOI: 10.1186/s13063-018-2959-y
Multicentre, double-blind, crossover trial to identify the Optimal Pathway for TreatIng neurOpathic paiN in Diabetes Mellitus (OPTION-DM): study protocol for a randomised controlled trial
Abstract
Background: The number of people with diabetes is growing rapidly. Diabetes can cause nerve damage leading to severe pain in the feet, legs and hands, which is known as diabetic peripheral neuropathic pain (DPNP). In the UK, the National Institute for Health and Care Excellence (NICE) recommends amitriptyline, duloxetine, pregabalin or gabapentin as initial treatment for DPNP. If this is not effective, adding one of the other drugs in combination with the first is recommended. NICE points out that these recommendations are not based on robust evidence. The OPTION-DM randomised controlled trial has been designed to address this evidence deficit, with the aims of determining the most clinically beneficial, cost-effective and tolerated treatment pathway for patients with DPNP.
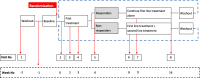
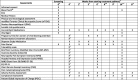
Methods/design: A multicentre, double-blind, centre-stratified, multi-period crossover study with equal allocation to sequences (1:1:1:1:1:1) of treatment pathways. Three hundred and ninety-two participants will be recruited from secondary care DPNP centres in the UK. There are three treatment pathways: amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline and duloxetine supplemented with pregabalin. All participants will receive all three pathways and randomisation will determine the order in which they are received. The primary outcome is the difference between 7-day average 24-h pain scores on an 11-point NRS scale measured during the final follow-up week of the treatment pathway. Secondary outcomes for efficacy, cost-effectiveness, safety, patient-perceived tolerability and subgroup analysis will be measured at week 6 and week 16 of each pathway.
Discussion: The study includes direct comparisons of the mainstay treatment for DPNP. This novel study is designed to examine treatment pathways and capture clinically relevant outcomes which will make the results generalisable to current clinical practice. The study will also provide information on health economic outcomes and will include a subgroup study to provide information on whether patient phenotypes predict response to treatment.
Trial registration: ISRCTN17545443 . Registered on 12 September 2016.
Keywords: Amitriptyline; Crossover trial; Diabetes; Duloxetine; Painful diabetic neuropathy; Pregabalin.
Conflict of interest statement
Ethics approval and consent to participate
The protocol was approved by Yorkshire and the Humber – Sheffield Research Ethics Committee (16/YH/0459) and received Medicines and Healthcare products Regulatory Agency (MHRA) clinical trials authorisation (21,304/0262/001). Written informed consent will be obtained from all participants prior to any study-specific procedures.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

