Safety and efficacy of nivolumab in combination with sunitinib or pazopanib in advanced or metastatic renal cell carcinoma: the CheckMate 016 study
- PMID: 30348216
- PMCID: PMC6196426
- DOI: 10.1186/s40425-018-0420-0
Safety and efficacy of nivolumab in combination with sunitinib or pazopanib in advanced or metastatic renal cell carcinoma: the CheckMate 016 study
Erratum in
-
Correction to: Safety and efficacy of nivolumab in combination with sunitinib or pazopanib in advanced or metastatic renal cell carcinoma: the CheckMate 016 study.J Immunother Cancer. 2019 Mar 14;7(1):73. doi: 10.1186/s40425-019-0559-3. J Immunother Cancer. 2019. PMID: 30871617 Free PMC article.
Abstract
Background: Combination treatment with immune checkpoint inhibitors and antiangiogenic drugs has shown encouraging preliminary antitumor activity across various tumor types including advanced or metastatic renal cell carcinoma (aRCC). The open-label, parallel-cohort, dose-escalation, phase I CheckMate 016 study evaluated the efficacy and safety of nivolumab in combination with antiangiogenic tyrosine kinase inhibitors or ipilimumab. Long-term outcomes from this study for the combination of nivolumab plus sunitinib or pazopanib in aRCC are presented.
Methods: Patients with aRCC received nivolumab plus either sunitinib (50 mg/day, 4 weeks on/2 weeks off; N + S) or pazopanib (800 mg/day; N + P) until progression/unacceptable toxicity. The nivolumab starting dose was 2 mg/kg every 3 weeks, with planned escalation to 5 mg/kg every 3 weeks. Primary endpoints were safety and tolerability; antitumor activity was a secondary endpoint.
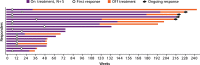
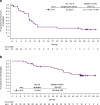
Results: Arm N + S enrolled 33 patients, 19 of whom were treatment-naïve; this arm advanced to the expansion phase. Median follow-up was 50.0 months. Patients experienced high frequencies of adverse events (AEs) including treatment-related AEs (100%), grade 3/4 treatment-related AEs (82%), and treatment-related AEs leading to discontinuation (39%). Investigator-assessed objective response rate (ORR) was 55% (18/33) and median progression-free survival (PFS) was 12.7 months. Median overall survival (OS) was not reached. Arm N + P enrolled 20 patients, all had ≥1 prior systemic therapy; this arm was closed due to dose-limiting toxicities and did not proceed to expansion. Median follow-up was 27.1 months. Patients treated with N + P experienced high frequencies of AEs including treatment-related AEs (100%), grade 3/4 treatment-related AEs (70%), and treatment-related AEs leading to discontinuation (25%). Investigator-assessed ORR was 45% (9/20) and median PFS was 7.2 months. Median OS was 27.9 months.
Conclusions: The addition of standard doses of sunitinib or pazopanib to nivolumab resulted in a high incidence of high-grade toxicities limiting future development of either combination regimen. While there was no adverse impact on response and the OS outcome was notable, the findings suggest that the success of combination regimens based on immune checkpoint inhibitors and antiangiogenic drugs may be dependent on careful selection of the antiangiogenic component and dose.
Trial registration: Clinicaltrials.gov identifier: NCT01472081 . Registered 16 November 2011.
Keywords: Antiangiogenic; Immune checkpoint inhibitor; Metastatic renal cell carcinoma; Nivolumab; Pazopanib; Sunitinib; Tyrosine kinase inhibitor.
Conflict of interest statement
Ethics approval and consent to participate
The CheckMate 016 study was approved by the local site-specific institutional review boards or an independent ethics committee and conducted in accordance with International Conference on Harmonisation Good Clinical Practice guidelines. Written informed consent was obtained from all patients based on Declaration of Helsinki principles before initiation of any study procedures. The trail was registered on
Consent for publication
Not applicable.
Competing interests
The following represents disclosure information provided by authors of this manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Choueiri TK, Halabi S, Sanford BL, Hahn O, Michaelson MD, Walsh MK, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the Alliance A031203 CABOSUN trial. J Clin Oncol. 2017;35(6):591–597. doi: 10.1200/JCO.2016.70.7398. - DOI - PMC - PubMed
-
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27(20):3312–3318. doi: 10.1200/JCO.2008.19.5511. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
