Avelumab in patients with previously treated metastatic adrenocortical carcinoma: phase 1b results from the JAVELIN solid tumor trial
- PMID: 30348224
- PMCID: PMC6198369
- DOI: 10.1186/s40425-018-0424-9
Avelumab in patients with previously treated metastatic adrenocortical carcinoma: phase 1b results from the JAVELIN solid tumor trial
Abstract
Background: We assessed the efficacy and safety of avelumab, an anti-programmed death ligand 1 (PD-L1) antibody, in patients with previously treated metastatic adrenocortical carcinoma (mACC).
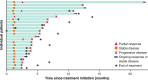
Methods: In this phase 1b expansion cohort, patients with mACC and prior platinum-based therapy received avelumab at 10 mg/kg intravenously every 2 weeks. Continuation of mitotane was permitted; however, mitotane levels during the study were not recorded. Tumor response was assessed by Response Evaluation Criteria In Solid Tumors v1.1.
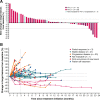
Results: Fifty patients received avelumab and were followed for a median of 16.5 months. Prior treatment included ≥2 lines in 74.0%; mitotane was continued in 50.0%. The objective response rate (ORR) was 6.0% (95% CI, 1.3% to 16.5%; partial response in 3 patients). Twenty-one patients (42.0%) had stable disease as best response (disease control rate, 48.0%). Median progression-free survival was 2.6 months (95% CI, 1.4 to 4.0), median overall survival (OS) was 10.6 months (95% CI, 7.4 to 15.0), and the 1-year OS rate was 43.4% (95% CI, 27.9% to 57.9%). In evaluable patients with PD-L1+ (n = 12) or PD-L1- (n = 30) tumors (≥5% tumor cell cutoff), ORR was 16.7% vs 3.3% (P = .192). Treatment-related adverse events (TRAEs) occurred in 82.0%; the most common were nausea (20.0%), fatigue (18.0%), hypothyroidism (14.0%), and pyrexia (14.0%). Grade 3 TRAEs occurred in 16.0%; no grade 4 to 5 TRAEs occurred. Twelve patients (24.0%) had an immune-related TRAE of any grade, which were grade 3 in 2 patients (4.0%): adrenal insufficiency (n = 1), and pneumonitis (n = 1).
Conclusions: Avelumab showed clinical activity and a manageable safety profile in patients with platinum-treated mACC.
Trial registration: Clinicaltrials.gov NCT01772004 ; registered January 21, 2013.
Keywords: Adrenocortical carcinoma; Avelumab; JAVELIN solid tumor; Neuroendocrine tumors; PD-L1; Phase 1.
Conflict of interest statement
Ethics approval and consent to participate
The trial was conducted in accordance with the ethics principles of the Declaration of Helsinki and the International Council for Harmonisation Guidelines on Good Clinical Practice. The protocol was approved by the institutional review board or independent ethics committee of each center. All patients provided written informed consent before enrollment.
Consent for publication
Not applicable.
Competing interests
C. Le Tourneau has served in consulting or advisory roles for Merck & Co., Bristol-Myers Squibb, Novartis, and AstraZeneca and has received honoraria from EMD Serono and AstraZeneca. C. Hoimes has served in consulting or advisory roles for Eisai and Seattle Genetics and has served on speakers bureaus for Bristol-Myers Squibb and Roche-Genentech. C. Zarwan has served in consulting or advisory roles for Perceptive Informatics and Revere Pharmaceutics. D.J. Wong has served in consulting or advisory roles for and has received honoraria from Bristol-Myers Squibb and has received research funding from Armo Biosciences, AstraZeneca/MedImmune, BioMed Valley Discoveries, Bristol-Myers Squibb, EMD Serono, KURA Oncology, Merck & Co., and Roche-Genentech. S. Bauer has served in consulting or advisory roles for Bayer, Blueprint Medicines, Deciphera, and Eli Lilly; has received honoraria from Bayer, Novartis, GSK, Pfizer, and PharmaMar; has received research funding from Blueprint Medicines, Incyte, and Novartis; and has received reimbursement for travel and accommodation expenses from PharmaMar. R. Claus has served in consulting or advisory roles for AbbVie, Gilead, Janssen Oncology, and Roche-Genentech and has received honoraria from Janssen Oncology and Roche. M. Wermke has served in consulting or advisory roles for AstraZeneca, Bristol-Myers Squibb, Novartis, and Roche-Genentech and has received research funding from Boehringer, Celgene, Novartis, and Pfizer. S. Hariharan reports employment with and stock ownership in Pfizer. A. von Heydebreck reports employment with and stock ownership in Merck KGaA. V. Kasturi reports employment with EMD Serono. V. Chand reports employment with EMD Serono and stock ownership in Bristol-Myers Squibb. J.L. Gulley has received research funding from Astellas Medivation, Bavarian Nordic, Celgene, EMD Serono, and Pfizer.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Fassnacht Martin, Johanssen Sarah, Quinkler Marcus, Bucsky Peter, Willenberg Holger S., Beuschlein Felix, Terzolo Massimo, Mueller Hans-Helge, Hahner Stefanie, Allolio Bruno. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma. Cancer. 2008;115(2):243–250. doi: 10.1002/cncr.24030. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
