Opposing roles of inter-α-trypsin inhibitor heavy chain 4 in recurrent pregnancy loss
- PMID: 30348621
- PMCID: PMC6286651
- DOI: 10.1016/j.ebiom.2018.10.029
Opposing roles of inter-α-trypsin inhibitor heavy chain 4 in recurrent pregnancy loss
Abstract
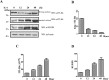
Background: The mechanism behind an increased risk of recurrent pregnancy loss (RPL) remains largely unknown. In our previous study, we identified that inter-α-trypsin inhibitor heavy chain 4 (ITI-H4) is highly expressed at a modified molecular weight of 36 kDa in serum derived from RPL patients. Yet, the precise molecular mechanism and pathways by which the short form of ITI-H4 carries out its function remain obscure.
Methods: Human sera and peripheral blood mononucleated cells (PBMCs) were collected from patients and normal controls to compare the expression levels of ITI-H4 and plasma kallikrein (KLKB1). Flow cytometric assay was performed to measure inflammatory markers in sera and culture supernatants. Furthermore, to investigate the functions of the two isoforms of ITI-H4, we performed migration, invasion, and proliferation assays.
Findings: In the current study, we showed that ITI-H4 as a biomarker of RPL could be regulated by KLKB1 through the IL-6 signaling cascade, indicating a novel regulatory system for inflammation in RPL. In addition, our study indicates that the two isoforms of ITI-H4 possess opposing functions on immune response, trophoblast invasion, and monocytes migration or proliferation.
Interpretation: The ITI-H4 (∆N688) might be a crucial inflammatory factor which contributes to the pathogenesis of RPL. Moreover, it is expected that this study would give some insights into potential functional mechanisms underlying RPL. FUND: This study was supported by the Ministry of Health & Welfare of the Republic of Korea (HI18C0378) through the Korea Health Industry Development Institute.
Keywords: Early pregnancy loss; ITI-H4; Immune tolerance; Migration and invasion; Plasma kallikrein.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures








References
-
- Lee J., Oh J., Choi E., Park I., Han C., Kim D.H. Differentially expressed genes implicated in unexplained recurrent spontaneous abortion. Int J Biochem Cell Biol. 2007;39(12):2265–2277. - PubMed
-
- Chaudhry K., Siccardi M. StatPearls Publishing LLC; Treasure Island FL: 2018. Blighted Ovum (Anembryonic Pregnancy). StatPearls. - PubMed
-
- Baek K.H. Aberrant gene expression associated with recurrent pregnancy loss. Mol Hum Reprod. 2004;10(5):291–297. - PubMed
-
- Caetano R., Ramisetty-Mikler S., Floyd L.R., McGrath C. The epidemiology of drinking among women of child-bearing age. Alcohol Clin Exp Res. 2006;30(6):1023–1030. - PubMed
-
- Pandey M.K., Rani R., Agrawal S. An update in recurrent spontaneous abortion. Arch Gynecol Obstet. 2005;272(2):95–108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

