The Reaction Mechanism of Metallo-β-Lactamases Is Tuned by the Conformation of an Active-Site Mobile Loop
- PMID: 30348667
- PMCID: PMC6325180
- DOI: 10.1128/AAC.01754-18
The Reaction Mechanism of Metallo-β-Lactamases Is Tuned by the Conformation of an Active-Site Mobile Loop
Abstract
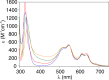
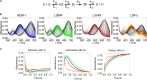
Carbapenems are "last resort" β-lactam antibiotics used to treat serious and life-threatening health care-associated infections caused by multidrug-resistant Gram-negative bacteria. Unfortunately, the worldwide spread of genes coding for carbapenemases among these bacteria is threatening these life-saving drugs. Metallo-β-lactamases (MβLs) are the largest family of carbapenemases. These are Zn(II)-dependent hydrolases that are active against almost all β-lactam antibiotics. Their catalytic mechanism and the features driving substrate specificity have been matter of intense debate. The active sites of MβLs are flanked by two loops, one of which, loop L3, was shown to adopt different conformations upon substrate or inhibitor binding, and thus are expected to play a role in substrate recognition. However, the sequence heterogeneity observed in this loop in different MβLs has limited the generalizations about its role. Here, we report the engineering of different loops within the scaffold of the clinically relevant carbapenemase NDM-1. We found that the loop sequence dictates its conformation in the unbound form of the enzyme, eliciting different degrees of active-site exposure. However, these structural changes have a minor impact on the substrate profile. Instead, we report that the loop conformation determines the protonation rate of key reaction intermediates accumulated during the hydrolysis of different β-lactams in all MβLs. This study demonstrates the existence of a direct link between the conformation of this loop and the mechanistic features of the enzyme, bringing to light an unexplored function of active-site loops on MβLs.
Keywords: New Delhi metallo-β-lactamase; antibiotic resistance; enzyme mechanism; enzyme structure; metallo-β-lactamase.
Copyright © 2018 American Society for Microbiology.
Figures




References
-
- Bonomo RA, Tolmasky M. 2007. Enzyme-mediated resistance to antibiotics: mechanisms, dissemination, and prospects for inhibition, 1st ed, vol 83 ASM Press, Washington, DC.
-
- Gonzalez MM, Kosmopoulou M, Mojica MF, Castillo V, Hinchliffe P, Pettinati I, Brem J, Schofield CJ, Mahler G, Bonomo RA, Llarrull LI, Spencer J, Vila AJ. 2015. Bisthiazolidines: a substrate-mimicking scaffold as an inhibitor of the NDM-1 carbapenemase. ACS Infect Dis 1:544–554. doi: 10.1021/acsinfecdis.5b00046. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

