Geranylgeraniol-induced Myogenic Differentiation of C2C12 Cells
- PMID: 30348697
- PMCID: PMC6365721
- DOI: 10.21873/invivo.11395
Geranylgeraniol-induced Myogenic Differentiation of C2C12 Cells
Abstract
Background: Geranylgeraniol (GGOH) is a C20 isoprenoid found in fruits, vegetables, and grains, including rice. As a food substance, GGOH is categorized as 'Generally Recognized as Safe'. GGOH is an intermediate product in the mevalonate pathway and acts as a precursor to geranylgeranyl pyrophosphate.
Materials and methods: C2C12 mouse myoblasts derived from muscle satellite cells were used. Quantitative reverse-transcriptase polymerase chain reaction, western blotting analysis, and immunocytochemical analysis were performed to respectively assess mRNA expression, protein levels, and the number of myofibers.
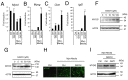
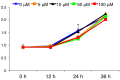
Results: GGOH reduced the expression levels of skeletal muscle atrophy-related ubiquitin ligases in myofibers derived from C2C12 cells. GGOH induced myogenic differentiation of C2C12 cells via geranylgeranylation. GGOH did not adversely affect the proliferation of C2C12 cells.
Conclusion: GGOH induces myoblast differentiation in C2C12 cells.
Keywords: C2C12 cells; Sarcopenia; geranylgeranylation; myogenesis; statin.
Copyright© 2018, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Figures

References
-
- Montarras D, L'Honore A, Buckingham M. Lying low but ready for action: the quiescent muscle satellite cell. FEBS J. 2013;280:4036–4050. - PubMed
-
- Kokabu S, Nakatomi C, Matsubara T, Ono Y, Addison WN, Lowery JW, Urata M, Hudnall AM, Hitomi S, Nakatomi M, Sato T, Osawa K, Yoda T, Rosen V, Jimi E. The transcriptional co-repressor TLE3 regulates myogenic differentiation by repressing the activity of the MyoD transcription factor. J Biol Chem. 2017;292:12885–12894. - PMC - PubMed
-
- Florini JR, Ewton DZ, Magri KA. Hormones, growth factors, and myogenic differentiation. Annu Rev Physiol. 1991;53:201–216. - PubMed
-
- Rosenblatt JD, Yong D, Parry DJ. Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve. 1994;17:608–613. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
