Phase II Study of Weekly Amrubicin for Refractory or Relapsed Small Cell Lung Cancer
- PMID: 30348719
- PMCID: PMC6365753
- DOI: 10.21873/invivo.11417
Phase II Study of Weekly Amrubicin for Refractory or Relapsed Small Cell Lung Cancer
Abstract
Background: Amrubicin hydrochloride is administered as second- or third-line therapy for small cell lung cancer, and is known to cause severe myelotoxicity. This study evaluated the efficacy and safety of weekly amrubicin for refractory/relapsed small cell lung cancer.
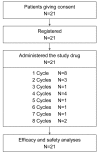
Patients and methods: A single-arm, open-label, multicenter, phase II study of weekly amrubicin was performed in 21 patients at seven centers in Japan from 2012 through 2015.
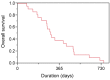
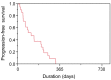
Results: A partial response (PR) was noted in one out of the first 18 patients. The study was terminated early according to the termination criteria in the protocol. In total, the response rate was 19% (no complete responses and four PRs) and the disease control rate was 81% (17/21). Median overall survival was 288 days (95% confidence interval(CI)=208-424 days), while median progression-free survival was 113 days (95% CI=45-202 days).
Conclusion: This study failed to demonstrate any efficacy of weekly amrubicin for refractory/relapsed small cell lung cancer.
Keywords: Amrubicin; phase II; refractory; relapsed; small cell lung cancer.
Copyright© 2018, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Figures
References
-
- Yana T, Negoro S, Takada M, Yokota S, Takada Y, Sugiura T, Yamamoto H, Sawa T, Kawahara M, Katakami N, Ariyoshi Y, Fukuoka M, West Japan Thoracic Oncology Group Phase II study of amrubicin in previously untreated patients with extensive-disease small cell lung cancer: West Japan Thoracic Oncology Group (WJTOG) study. Invest New Drugs. 2007;25:253–258. - PubMed
-
- Onoda S, Masuda N, Seto T, Eguchi K, Takiguchi Y, Isobe H, Okamoto H, Ogura T, Yokoyama A, Seki N, Asaka-Amano Y, Harada M, Tagawa A, Kunikane H, Yokoba M, Uematsu K, Kuriyama T, Kuroiwa Y, Watanabe K, Thoracic Oncology Research Group Study 0301 Phase II trial of amrubicin for treatment of refractory or relapsed small-cell lung cancer: Thoracic Oncology Research Group Study 0301. J Clin Oncol. 2006;24:5448–5453. - PubMed
-
- Inoue A, Sugawara S, Yamazaki K, Maemondo M, Suzuki T, Gomi K, Takanashi S, Inoue C, Inage M, Yokouchi H, Watanabe H, Tsukamoto T, Saijo Y, Ishimoto O, Hommura F, Nukiwa T. Randomized phase II trial comparing amrubicin with topotecan in patients with previously treated small-cell lung cancer: North Japan Lung Cancer Study Group Trial 0402. J Clin Oncol. 2008;26:5401–5406. - PubMed
-
- Ettinger DS, Jotte R, Lorigan P, Gupta V, Garbo L, Alemany C, Conkling P, Spigel DR, Dudek AZ, Shah C, Salgia R, McNally R, Renschler MF, Oliver JW. Phase II study of amrubicin as second-line therapy in patients with platinum-refractory small-cell lung cancer. J Clin Oncol. 2010;28:2598–2603. - PubMed
-
- Jotte R, Conkling P, Reynolds C, Galsky MD, Klein L, Fitzgibbons JF, McNally R, Renschler MF, Oliver JW. Randomized phase II trial of single-agent amrubicin or topotecan as second-line treatment in patients with small-cell lung cancer sensitive to first-line platinum-based chemotherapy. J Clin Oncol. 2011;29:287–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials