Lipoxin B4 Enhances Human Memory B Cell Antibody Production via Upregulating Cyclooxygenase-2 Expression
- PMID: 30348736
- PMCID: PMC6246818
- DOI: 10.4049/jimmunol.1700503
Lipoxin B4 Enhances Human Memory B Cell Antibody Production via Upregulating Cyclooxygenase-2 Expression
Abstract
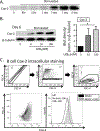
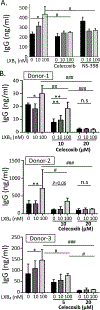
Vaccination has been the most effective way to prevent or reduce infectious diseases; examples include the eradication of smallpox and attenuation of tetanus and measles. However, there is a large segment of the population that responds poorly to vaccines, in part because they are immunocompromised because of disease, age, or pharmacologic therapy and are unable to generate long-term protection. Specialized proresolving mediators are endogenously produced lipids that have potent proresolving and anti-inflammatory activities. Lipoxin B4 (LXB4) is a member of the lipoxin family, with its proresolving effects shown in allergic airway inflammation. However, its effects on the adaptive immune system, especially on human B cells, are not known. In this study, we investigated the effects of LXB4 on human B cells using cells from healthy donors and donors vaccinated against influenza virus in vitro. LXB4 promoted IgG Ab production in memory B cells and also increased the number of IgG-secreting B cells. LXB4 enhanced expression of two key transcription factors involved in plasma cell differentiation, BLIMP1 and XBP1. Interestingly, LXB4 increased expression of cyclooxygenase-2 (COX2), an enzyme that is required for efficient B cell Ab production. The effects of LXB4 are at least partially COX2-dependent as COX2 inhibitors attenuated LXB4-stimulated BLIMP1 and Xpb-1 expression as well as IgG production. Thus, our study reveals for the first time, to our knowledge, that LXB4 boosts memory B cell activation through COX2 and suggests that LXB4 can serve as a new vaccine adjuvant.
Copyright © 2018 by The American Association of Immunologists, Inc.
Figures



References
-
- Mulligan MJ, Bernstein DI, Winokur P, Rupp R, Anderson E, Rouphael N, Dickey M, Stapleton JT, Edupuganti S, Spearman P, Ince D, Noah DL, Hill H, Bellamy AR, and Group DHNVS 2014. Serological responses to an avian influenza A/H7N9 vaccine mixed at the point-of-use with MF59 adjuvant: a randomized clinical trial. JAMA 312: 1409–1419. - PubMed
-
- Serhan CN 2007. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol 25: 101–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

