Essential Roles of the sppRA Fructose-Phosphate Phosphohydrolase Operon in Carbohydrate Metabolism and Virulence Expression by Streptococcus mutans
- PMID: 30348833
- PMCID: PMC6304665
- DOI: 10.1128/JB.00586-18
Essential Roles of the sppRA Fructose-Phosphate Phosphohydrolase Operon in Carbohydrate Metabolism and Virulence Expression by Streptococcus mutans
Abstract
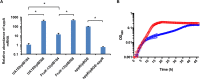
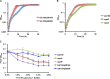
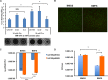
The dental caries pathogen Streptococcus mutans can ferment a variety of sugars to produce organic acids. Exposure of S. mutans to certain nonmetabolizable carbohydrates, such as xylitol, impairs growth and can cause cell death. Recently, the presence of a sugar-phosphate stress in S. mutans was demonstrated using a mutant lacking 1-phosphofructokinase (FruK) that accumulates fructose-1-phosphate (F-1-P). Here, we studied an operon in S. mutans, sppRA, which was highly expressed in the fruK mutant. Biochemical characterization of a recombinant SppA protein indicated that it possessed hexose-phosphate phosphohydrolase activity, with preferences for F-1-P and, to a lesser degree, fructose-6-phosphate (F-6-P). SppA activity was stimulated by Mg2+ and Mn2+ but inhibited by NaF. SppR, a DeoR family regulator, repressed the expression of the sppRA operon to minimum levels in the absence of the fructose-derived metabolite F-1-P and likely also F-6-P. The accumulation of F-1-P, as a result of growth on fructose, not only induced sppA expression, but it significantly altered biofilm maturation through increased cell lysis and enhanced extracellular DNA release. Constitutive expression of sppA, via a plasmid or by deleting sppR, greatly alleviated fructose-induced stress in a fruK mutant, enhanced resistance to xylitol, and reversed the effects of fructose on biofilm formation. Finally, by identifying three additional putative phosphatases that are capable of promoting sugar-phosphate tolerance, we show that S. mutans is capable of mounting a sugar-phosphate stress response by modulating the levels of certain glycolytic intermediates, functions that are interconnected with the ability of the organism to manifest key virulence behaviors.IMPORTANCEStreptococcus mutans is a major etiologic agent for dental caries, primarily due to its ability to form biofilms on the tooth surface and to convert carbohydrates into organic acids. We have discovered a two-gene operon in S. mutans that regulates fructose metabolism by controlling the levels of fructose-1-phosphate, a potential signaling compound that affects bacterial behaviors. With fructose becoming increasingly common and abundant in the human diet, we reveal the ways that fructose may alter bacterial development, stress tolerance, and microbial ecology in the oral cavity to promote oral diseases.
Keywords: biofilm; fructose metabolism; sugar-phosphate phosphohydrolase; sugar-phosphate stress; xylitol.
Copyright © 2018 American Society for Microbiology.
Figures

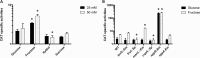


Similar articles
-
Coordinated Regulation of the EIIMan and fruRKI Operons of Streptococcus mutans by Global and Fructose-Specific Pathways.Appl Environ Microbiol. 2017 Oct 17;83(21):e01403-17. doi: 10.1128/AEM.01403-17. Print 2017 Nov 1. Appl Environ Microbiol. 2017. PMID: 28821551 Free PMC article.
-
Involvement of an inducible fructose phosphotransferase operon in Streptococcus gordonii biofilm formation.J Bacteriol. 2003 Nov;185(21):6241-54. doi: 10.1128/JB.185.21.6241-6254.2003. J Bacteriol. 2003. PMID: 14563858 Free PMC article.
-
Characterization of the Trehalose Utilization Operon in Streptococcus mutans Reveals that the TreR Transcriptional Regulator Is Involved in Stress Response Pathways and Toxin Production.J Bacteriol. 2018 May 24;200(12):e00057-18. doi: 10.1128/JB.00057-18. Print 2018 Jun 15. J Bacteriol. 2018. PMID: 29632089 Free PMC article.
-
The VicRK Two-Component System Regulates Streptococcus mutans Virulence.Curr Issues Mol Biol. 2019;32:167-200. doi: 10.21775/cimb.032.167. Epub 2019 Jun 5. Curr Issues Mol Biol. 2019. PMID: 31166172 Review.
-
Dlt operon regulates physiological function and cariogenic virulence in Streptococcus mutans.Future Microbiol. 2023 Mar;18:225-233. doi: 10.2217/fmb-2022-0165. Epub 2023 Apr 25. Future Microbiol. 2023. PMID: 37097048 Review.
Cited by
-
Potential of Prebiotic D-Tagatose for Prevention of Oral Disease.Front Cell Infect Microbiol. 2021 Nov 5;11:767944. doi: 10.3389/fcimb.2021.767944. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34804997 Free PMC article.
-
Glucose Phosphotransferase System Modulates Pyruvate Metabolism, Bacterial Fitness, and Microbial Ecology in Oral Streptococci.J Bacteriol. 2023 Jan 26;205(1):e0035222. doi: 10.1128/jb.00352-22. Epub 2022 Dec 5. J Bacteriol. 2023. PMID: 36468868 Free PMC article.
-
CcpA and CodY Regulate CRISPR-Cas System of Streptococcus mutans.Microbiol Spectr. 2023 Aug 17;11(4):e0182623. doi: 10.1128/spectrum.01826-23. Epub 2023 Jun 27. Microbiol Spectr. 2023. PMID: 37367300 Free PMC article.
-
Molecular mechanisms controlling fructose-specific memory and catabolite repression in lactose metabolism by Streptococcus mutans.Mol Microbiol. 2021 Jan;115(1):70-83. doi: 10.1111/mmi.14597. Epub 2020 Sep 25. Mol Microbiol. 2021. PMID: 32881130 Free PMC article.
-
A single system detects and protects the beneficial oral bacterium Streptococcus sp. A12 from a spectrum of antimicrobial peptides.Mol Microbiol. 2021 Jul;116(1):211-230. doi: 10.1111/mmi.14703. Epub 2021 Feb 25. Mol Microbiol. 2021. PMID: 33590560 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

