Phosphatidylinositol 4,5-bisphosphate (PIP2) regulates KCNQ3 K+ channels by interacting with four cytoplasmic channel domains
- PMID: 30348901
- PMCID: PMC6302169
- DOI: 10.1074/jbc.RA118.005401
Phosphatidylinositol 4,5-bisphosphate (PIP2) regulates KCNQ3 K+ channels by interacting with four cytoplasmic channel domains
Abstract
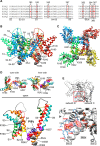
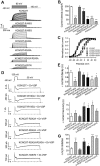
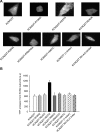
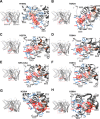
Phosphatidylinositol 4,5-bisphosphate (PIP2) in the plasma membrane regulates the function of many ion channels, including M-type (potassium voltage-gated channel subfamily Q member (KCNQ), Kv7) K+ channels; however, the molecular mechanisms involved remain unclear. To this end, we here focused on the KCNQ3 subtype that has the highest apparent affinity for PIP2 and performed extensive mutagenesis in regions suggested to be involved in PIP2 interactions among the KCNQ family. Using perforated patch-clamp recordings of heterologously transfected tissue culture cells, total internal reflection fluorescence microscopy, and the zebrafish (Danio rerio) voltage-sensitive phosphatase to deplete PIP2 as a probe, we found that PIP2 regulates KCNQ3 channels through four different domains: 1) the A-B helix linker that we previously identified as important for both KCNQ2 and KCNQ3, 2) the junction between S6 and the A helix, 3) the S2-S3 linker, and 4) the S4-S5 linker. We also found that the apparent strength of PIP2 interactions within any of these domains was not coupled to the voltage dependence of channel activation. Extensive homology modeling and docking simulations with the WT or mutant KCNQ3 channels and PIP2 were consistent with the experimental data. Our results indicate that PIP2 modulates KCNQ3 channel function by interacting synergistically with a minimum of four cytoplasmic domains.
Keywords: KCNQ; M current; PIP2; ion channel gating; ion channel modulation; lipid signaling; neuroscience; phospholipid; potassium channel; signal transduction; structure-function.
© 2018 Choveau et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
-
- Loussouarn G., Park K. H., Bellocq C., Baró I., Charpentier F., and Escande D. (2003) Phosphatidylinositol-4,5-bisphosphate, PIP2, controls KCNQ1/KCNE1 voltage-gated potassium channels: a functional homology between voltage-gated and inward rectifier K+ channels. EMBO J. 22, 5412–5421 10.1093/emboj/cdg526 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

