It takes a dimer to tango: Oligomeric small heat shock proteins dissociate to capture substrate
- PMID: 30348902
- PMCID: PMC6314120
- DOI: 10.1074/jbc.RA118.005421
It takes a dimer to tango: Oligomeric small heat shock proteins dissociate to capture substrate
Abstract
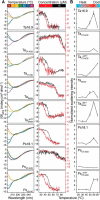
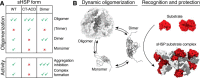
Small heat-shock proteins (sHsps) are ubiquitous molecular chaperones, and sHsp mutations or altered expression are linked to multiple human disease states. sHsp monomers assemble into large oligomers with dimeric substructure, and the dynamics of sHsp oligomers has led to major questions about the form that captures substrate, a critical aspect of their mechanism of action. We show here that substructural dimers of two plant dodecameric sHsps, Ta16.9 and homologous Ps18.1, are functional units in the initial encounter with unfolding substrate. We introduced inter-polypeptide disulfide bonds at the two dodecameric interfaces, dimeric and nondimeric, to restrict how their assemblies can dissociate. When disulfide-bonded at the nondimeric interface, mutants of Ta16.9 and Ps18.1 (TaCT-ACD and PsCT-ACD) were inactive but, when reduced, had WT-like chaperone activity, demonstrating that dissociation at nondimeric interfaces is essential for sHsp activity. Moreover, the size of the TaCT-ACD and PsCT-ACD covalent unit defined a new tetrahedral geometry for these sHsps, different from that observed in the Ta16.9 X-ray structure. Importantly, oxidized Tadimer (disulfide bonded at the dimeric interface) exhibited greatly enhanced ability to protect substrate, indicating that strengthening the dimeric interface increases chaperone efficiency. Temperature-induced size and secondary structure changes revealed that folded sHsp dimers interact with substrate and that dimer stability affects chaperone efficiency. These results yield a model in which sHsp dimers capture substrate before assembly into larger, heterogeneous sHsp-substrate complexes for substrate refolding or degradation, and suggest that tuning the strength of the dimer interface can be used to engineer sHsp chaperone efficiency.
Keywords: chaperone; chaperone efficiency; disulfides; dynamic light scattering (DLS); native mass spectrometry; oligomerization; protein design; protein engineering; protein folding; protein stability; small heat shock protein (sHsp); small-angle X-ray scattering (SAXS); stress response; substrate recognition; thermal stability.
© 2018 Santhanagopalan et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
-
- Santhanagopalan I., Basha E., Ballard K. N., Bopp N. E., and Vierling E. (2015) Model chaperones: Small heat shock proteins from plants. in The Big Book on Small Heat Shock Proteins (Tanguay R. M., and Hightower L. E., eds), pp. 119–153, Springer International Publishing, Cham, Switzerland: 10.1007/978-3-319-16077-1 - DOI
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

