Heterozygous deletion of chromosome 17p renders prostate cancer vulnerable to inhibition of RNA polymerase II
- PMID: 30349055
- PMCID: PMC6197287
- DOI: 10.1038/s41467-018-06811-z
Heterozygous deletion of chromosome 17p renders prostate cancer vulnerable to inhibition of RNA polymerase II
Abstract
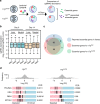
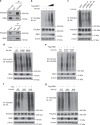
Heterozygous deletion of chromosome 17p (17p) is one of the most frequent genomic events in human cancers. Beyond the tumor suppressor TP53, the POLR2A gene encoding the catalytic subunit of RNA polymerase II (RNAP2) is also included in a ~20-megabase deletion region of 17p in 63% of metastatic castration-resistant prostate cancer (CRPC). Using a focused CRISPR-Cas9 screen, we discovered that heterozygous loss of 17p confers a selective dependence of CRPC cells on the ubiquitin E3 ligase Ring-Box 1 (RBX1). RBX1 activates POLR2A by the K63-linked ubiquitination and thus elevates the RNAP2-mediated mRNA synthesis. Combined inhibition of RNAP2 and RBX1 profoundly suppress the growth of CRPC in a synergistic manner, which potentiates the therapeutic effectivity of the RNAP2 inhibitor, α-amanitin-based antibody drug conjugate (ADC). Given the limited therapeutic options for CRPC, our findings identify RBX1 as a potentially therapeutic target for treating human CRPC harboring heterozygous deletion of 17p.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
- R21 CA213535/CA/NCI NIH HHS/United States
- R21CA213535/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)/International
- R01 CA203737/CA/NCI NIH HHS/United States
- R01 CA222251/CA/NCI NIH HHS/United States
- R01CA203737/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)/International
- R01CA222251/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)/International
- R01 GM115622/GM/NIGMS NIH HHS/United States
- R01 CA206366/CA/NCI NIH HHS/United States
- R01 CA211861/CA/NCI NIH HHS/United States
- R01CA211861/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)/International
- R01CA206366/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)/International
- R01 CA207701/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

