High bone turnover status as a risk factor in symptomatic hypocalcemia following denosumab treatment in a male patient with osteoporosis
- PMID: 30349211
- PMCID: PMC6183698
- DOI: 10.2147/CIA.S180614
High bone turnover status as a risk factor in symptomatic hypocalcemia following denosumab treatment in a male patient with osteoporosis
Abstract
Denosumab is a fully human monoclonal antibody against the receptor activator of nuclear factor-κB ligand (RANKL) that is used for the treatment of osteoporosis. Denosumab-induced hypocalcemia is a rare but important adverse event, which is usually asymptomatic in patients with osteoporosis. It is also known that hypocalcemia is common in patients with bone metastases and severe renal impairment. Here we report a case of symptomatic hypocalcemia following administration of 60 mg of denosumab in a patient with high bone turnover and no renal impairment (estimated glomerular filtration rate [eGFR], 71 mL/min), despite prophylactic oral vitamin D administration. This report supports our observation that there is a risk of protracted and marked denosumab-induced hypocalcemia in patients with high bone turnover, irrespective of their degree of renal impairment.
Keywords: denosumab; high bone turnover; no renal impairment; symptomatic hypocalcemia.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
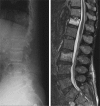

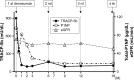
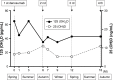
References
-
- Mccurry J. Japan will be model for future super-ageing societies. Lancet. 2015;386(10003):1523. - PubMed
-
- Cummings SR, San Martin J, et al. FREEDOM Trial Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–765. - PubMed
-
- Goldhahn J, Féron JM, Kanis J, et al. Implications for fracture healing of current and new osteoporosis treatments: an ESCEO consensus paper. Calcif Tissue Int. 2012;90(5):343–353. - PubMed
-
- Adami S, Libanati C, Boonen S, et al. Denosumab Treatment in Postmenopausal. J Bone Jt Surgery Incorporated. 2012;94-A(23):1–7. - PubMed
-
- Smyth B, Ong S. Severe hypocalcaemia and hypophosphataemia following intravenous iron and denosumab: a novel drug interaction. Intern Med J. 2016;46(3):360–363. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

