Nanocomposite plasters for the treatment of superficial tumors by chemo-photothermal combination therapy
- PMID: 30349247
- PMCID: PMC6188105
- DOI: 10.2147/IJN.S170209
Nanocomposite plasters for the treatment of superficial tumors by chemo-photothermal combination therapy
Abstract
Introduction: Novel nanomedical systems are being developed as multiple therapeutic modalities because the combinational therapies for cancer on a single platform can have larger chance to address tumor heterogeneity and drug resistance than any mono-therapeutic modality.
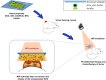
Methods: In this study, photothermal therapy (PTT) and chemotherapy (CT) were combined to treat squamous cell carcinoma by using a novel type of noninvasive plaster composed of carboxylated-reduced graphene oxide (rGO-COOH), gold nanorods (Au NRs), and doxorubicin (DOX). Firstly, DOX was loaded onto rGO-COOH to form DOX_rGO-COOH. Then, the obtained DOX_rGO-COOH and Au NRs were co-assembled to obtain nanocomposite multilayer. rGO-COOH and Au NRs were combined together to obtain high light-to-heat conversion efficiency. Using them as photothermal agents for PTT and using DOX in rGO-COOH as an anticancer drug for CT, their synergistic combination therapy could be applicable practically.
Results: As a result, DOX_rGO-COOH/Au NRs showed higher photothermal effects than that showed by rGO-COOH or Au NRs alone. It also showed higher therapeutic effects than DOX_rGO-COOH (for CT) or Au nr (for PTT) alone. Moreover, the system can repeatedly produce heat and simultaneously stimulate the release of the encapsulated anticancer drug into the tumor upon being irradiated by near-infrared laser. In vivo experiments demonstrated that the squamous cell carcinoma-bearing mice treated with DOX_rGO-COOH/Au NRs were healthy for more than 60 days without tumor recurrence.
Conclusion: The as-developed DOX_rGO-COOH/Au NRs plaster could be an effective, convenient, and noninvasive treatment option for treating superficial tumors.
Keywords: Au nanorods; carboxylated-reduced graphene oxide; doxorubicin; nanocomposite plaster; superficial tumor.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures







Similar articles
-
The polyvinylpyrrolidone functionalized rGO/Bi2S3 nanocomposite as a near-infrared light-responsive nanovehicle for chemo-photothermal therapy of cancer.Nanoscale. 2016 Jun 2;8(22):11531-42. doi: 10.1039/c6nr01543c. Nanoscale. 2016. PMID: 27203525
-
cRGD-Conjugated Fe3O4@PDA-DOX Multifunctional Nanocomposites for MRI and Antitumor Chemo-Photothermal Therapy.Int J Nanomedicine. 2019 Dec 5;14:9631-9645. doi: 10.2147/IJN.S222797. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31824156 Free PMC article.
-
Nitroxide-radicals-modified gold nanorods for in vivo CT/MRI-guided photothermal cancer therapy.Int J Nanomedicine. 2018 Nov 6;13:7123-7134. doi: 10.2147/IJN.S171804. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30464463 Free PMC article.
-
Functionalized Reduced Graphene Oxide as a Versatile Tool for Cancer Therapy.Int J Mol Sci. 2021 Mar 15;22(6):2989. doi: 10.3390/ijms22062989. Int J Mol Sci. 2021. PMID: 33804239 Free PMC article. Review.
-
Applications of Inorganic Nanomaterials in Photothermal Therapy Based on Combinational Cancer Treatment.Int J Nanomedicine. 2020 Mar 19;15:1903-1914. doi: 10.2147/IJN.S239751. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32256067 Free PMC article. Review.
Cited by
-
Membrane engineering of cell membrane biomimetic nanoparticles for nanoscale therapeutics.Clin Transl Med. 2021 Feb;11(2):e292. doi: 10.1002/ctm2.292. Clin Transl Med. 2021. PMID: 33635002 Free PMC article. Review.
-
Cell membrane camouflaged nanoparticles: a new biomimetic platform for cancer photothermal therapy.Int J Nanomedicine. 2019 Jun 17;14:4431-4448. doi: 10.2147/IJN.S200284. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31354269 Free PMC article. Review.
-
Graphene-based nanomaterials for cancer therapy and anti-infections.Bioact Mater. 2022 Feb 5;14:335-349. doi: 10.1016/j.bioactmat.2022.01.045. eCollection 2022 Aug. Bioact Mater. 2022. PMID: 35386816 Free PMC article. Review.
References
-
- Chen MC, Lin ZW, Ling MH. Near-infrared light-activatable microneedle system for treating superficial tumors by combination of chemotherapy and photothermal therapy. ACS Nano. 2016;10(1):93–101. - PubMed
-
- Abbas M, Zou Q, Li S, Yan X. Self-assembled peptide- and protein-based nanomaterials for antitumor photodynamic and photothermal therapy. Adv Mater. 2017;29(12):1605021. - PubMed
-
- Robinson JT, Tabakman SM, Liang Y, et al. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J Am Chem Soc. 2011;133(17):6825–6831. - PubMed
-
- Zou Q, Abbas M, Zhao L, Li S, Shen G, Yan X. Biological photothermal nanodots based on self-assembly of peptide-porphyrin conjugates for antitumor therapy. J Am Chem Soc. 2017;139(5):1921–1927. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

