Severe toxicity to capecitabine due to a new variant at a donor splicing site in the dihydropyrimidine dehydrogenase (DPYD) gene
- PMID: 30349384
- PMCID: PMC6190816
- DOI: 10.2147/CMAR.S174470
Severe toxicity to capecitabine due to a new variant at a donor splicing site in the dihydropyrimidine dehydrogenase (DPYD) gene
Abstract
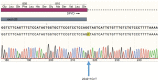
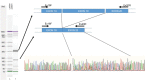
Severe, life-threatening adverse reactions to capecitabine sometimes occur in the treatment of solid tumors. Screening for dihydropyrimidine dehydrogenase (DPYD) deficiency is encouraged before start of treatment, but the genetic variants that are commonly analyzed often fail to explain toxicities seen in clinical practice. Here we describe the case of a 79-year-old Caucasian female with breast cancer who presented with life-threatening, rapidly increasing toxicity after 1 week of treatment with capecitabine and for whom routine genetic DPYD test resulted negative. DPYD exon sequencing found variant c.2242+1G>T at the donor splicing site of exon 19. This variant is responsible for skipping of exon 19 and subsequent generation of a non-functional DPYD enzyme. This variant has not been described previously but was found in three other members of the patient's family. With this case, we show that exon sequencing of DPYD in patients who experience marked toxicity to fluoropyrimidines and test negative for commonly evaluated variants can prove extremely useful for identifying new genetic variants and better explain adverse reactions causality.
Keywords: adverse drug reaction; breast cancer; fluoropyrimidine; pharmacogenetics.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

Similar articles
-
DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis.Lancet Oncol. 2018 Nov;19(11):1459-1467. doi: 10.1016/S1470-2045(18)30686-7. Epub 2018 Oct 19. Lancet Oncol. 2018. PMID: 30348537
-
Severe fluoropyrimidine toxicity due to novel and rare DPYD missense mutations, deletion and genomic amplification affecting DPD activity and mRNA splicing.Biochim Biophys Acta Mol Basis Dis. 2017 Mar;1863(3):721-730. doi: 10.1016/j.bbadis.2016.12.010. Epub 2016 Dec 24. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 28024938
-
Uncommon dihydropyrimidine dehydrogenase mutations and toxicity by fluoropyrimidines: a lethal case with a new variant.Pharmacogenomics. 2016;17(1):5-9. doi: 10.2217/pgs.15.146. Epub 2015 Dec 14. Pharmacogenomics. 2016. PMID: 26651493
-
DPYD Exon 4 Deletion Associated with Fluoropyrimidine Toxicity and Importance of Copy Number Variation.Curr Oncol. 2023 Jan 4;30(1):663-672. doi: 10.3390/curroncol30010051. Curr Oncol. 2023. PMID: 36661700 Free PMC article. Review.
-
[Dihydropyrimidine déhydrogenase (DPD) deficiency screening and securing of fluoropyrimidine-based chemotherapies: Update and recommendations of the French GPCO-Unicancer and RNPGx networks].Bull Cancer. 2018 Apr;105(4):397-407. doi: 10.1016/j.bulcan.2018.02.001. Epub 2018 Feb 24. Bull Cancer. 2018. PMID: 29486921 Review. French.
Cited by
-
Consensus of experts from the Spanish Pharmacogenetics and Pharmacogenomics Society and the Spanish Society of Medical Oncology for the genotyping of DPYD in cancer patients who are candidates for treatment with fluoropyrimidines.Clin Transl Oncol. 2022 Mar;24(3):483-494. doi: 10.1007/s12094-021-02708-4. Epub 2021 Nov 13. Clin Transl Oncol. 2022. PMID: 34773566 Free PMC article.
-
New DPYD variants causing DPD deficiency in patients treated with fluoropyrimidine.Cancer Chemother Pharmacol. 2020 Jul;86(1):45-54. doi: 10.1007/s00280-020-04093-1. Epub 2020 Jun 11. Cancer Chemother Pharmacol. 2020. PMID: 32529295
-
DPYD Exome, mRNA Expression and Uracil Levels in Early Severe Toxicity to Fluoropyrimidines: An Extreme Phenotype Approach.J Pers Med. 2021 Aug 13;11(8):792. doi: 10.3390/jpm11080792. J Pers Med. 2021. PMID: 34442436 Free PMC article.
-
Clinical Implementation of Rare and Novel DPYD Variants for Personalizing Fluoropyrimidine Treatment: Challenges and Opportunities.Int J Biol Sci. 2024 Jul 2;20(10):3742-3759. doi: 10.7150/ijbs.97686. eCollection 2024. Int J Biol Sci. 2024. PMID: 39113696 Free PMC article. Review.
References
-
- Mercier C, Ciccolini J. Profiling dihydropyrimidine dehydrogenase deficiency in patients with cancer undergoing 5-fluorouracil/capecitabine therapy. Clin Colorectal Cancer. 2006;6(4):288–296. - PubMed
-
- Saif MW, Katirtzoglou NA, Syrigos KN. Capecitabine: an overview of the side effects and their management. Anticancer Drugs. 2008;19(5):447–464. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

