The Genome Sequence of the Wild Tomato Solanum pimpinellifolium Provides Insights Into Salinity Tolerance
- PMID: 30349549
- PMCID: PMC6186997
- DOI: 10.3389/fpls.2018.01402
The Genome Sequence of the Wild Tomato Solanum pimpinellifolium Provides Insights Into Salinity Tolerance
Abstract
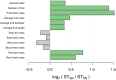
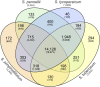
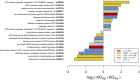
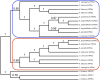
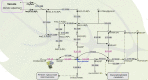
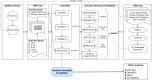
Solanum pimpinellifolium, a wild relative of cultivated tomato, offers a wealth of breeding potential for desirable traits such as tolerance to abiotic and biotic stresses. Here, we report the genome assembly and annotation of S. pimpinellifolium 'LA0480.' Moreover, we present phenotypic data from one field experiment that demonstrate a greater salinity tolerance for fruit- and yield-related traits in S. pimpinellifolium compared with cultivated tomato. The 'LA0480' genome assembly size (811 Mb) and the number of annotated genes (25,970) are within the range observed for other sequenced tomato species. We developed and utilized the Dragon Eukaryotic Analyses Platform (DEAP) to functionally annotate the 'LA0480' protein-coding genes. Additionally, we used DEAP to compare protein function between S. pimpinellifolium and cultivated tomato. Our data suggest enrichment in genes involved in biotic and abiotic stress responses. To understand the genomic basis for these differences in S. pimpinellifolium and S. lycopersicum, we analyzed 15 genes that have previously been shown to mediate salinity tolerance in plants. We show that S. pimpinellifolium has a higher copy number of the inositol-3-phosphate synthase and phosphatase genes, which are both key enzymes in the production of inositol and its derivatives. Moreover, our analysis indicates that changes occurring in the inositol phosphate pathway may contribute to the observed higher salinity tolerance in 'LA0480.' Altogether, our work provides essential resources to understand and unlock the genetic and breeding potential of S. pimpinellifolium, and to discover the genomic basis underlying its environmental robustness.
Keywords: Solanum pimpinellifolium; genome analysis; inositol 3-phosphate synthase; salinity tolerance; wild tomato.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

