Regulatory Role of Silicon in Mediating Differential Stress Tolerance Responses in Two Contrasting Tomato Genotypes Under Osmotic Stress
- PMID: 30349552
- PMCID: PMC6187069
- DOI: 10.3389/fpls.2018.01475
Regulatory Role of Silicon in Mediating Differential Stress Tolerance Responses in Two Contrasting Tomato Genotypes Under Osmotic Stress
Abstract
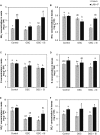
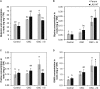
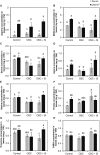
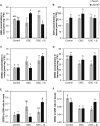
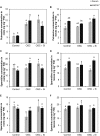
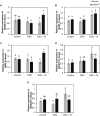
Previous studies have shown the role of silicon (Si) in mitigating the adverse effect of drought stress in different crop species. However, data are lacking on a comparison of drought tolerant and drought sensitive crop cultivars in response to Si nutrition. Therefore, the aim of this study was to elucidate the mechanism (s) by which two contrasting tomato genotypes respond to Si nutrition under osmotic stress condition. Two tomato lines contrasting in their response to drought stress were hydroponically grown under polyethylene glycol (PEG, 6000) and two regimes of Si (0 and 1.5 mM). Metabolite profiling was performed in two lines. Growth and relevant physiological parameters, and expression levels of selected genes were also measured. Si application resulted in improved osmotic stress tolerance in both drought tolerant line LA0147 and drought sensitive line FERUM. In the drought tolerant line, Si enhanced uptake of sulfur (S) and ammonium ( ) which led to a significantly higher production of amino acids arginine, methionine, serine, and glycine. While in the drought sensitive line, Si significantly increased production of amino acids proline and GABA which further lowered the level of GSSG to GSH ratio and thus balanced the redox homeostasis under osmotic stress. The higher significant production of amino acids arginine, methionine, GABA, and proline enhanced production of free polyamines putrescine and spermidine and improved osmotic stress tolerance. Therefore, we conclude that Si distinctively regulated osmotic stress tolerance in two contrasting tomato genotypes by differential accumulation of relevant amino acids which eventually led to enhanced polyamine metabolism.
Keywords: amino acids; metabolites; mineral nutrition and redox state; polyamines; tomato.
Figures


References
-
- Amin M., Ahmad R., Ali A., Hussain I., Mahmood R., Aslam M., et al. (2016). Influence of silicon fertilization on maize performance under limited water supply. Silicon 10 177–183. 10.1007/s12633-015-9372-x - DOI
LinkOut - more resources
Full Text Sources

