Contact Dermatitis Caused by Dermabond Advanced Use
- PMID: 30349768
- PMCID: PMC6191225
- DOI: 10.1097/GOX.0000000000001841
Contact Dermatitis Caused by Dermabond Advanced Use
Abstract
Background: Dermabond Advanced (DBA) has been widely used globally; however, severe contact dermatitis (CD) can be a serious adverse effect of DBA use. In this study, we investigated the characterization and incidence rate of CD after using DBA and the safe use of DBA.
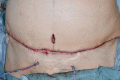
Methods: One hundred consecutive patients who underwent skin closure with DBA were investigated. All patients were women undergoing breast reconstruction. DBA was applied to their trunk and limbs following reconstruction.
Results: Seven patients (7%) presented with CD. Of these, 4 patients exhibited CD after the second DBA use; sensitization influence by the first DBA use was considered. One of 3 patients presenting with CD after the first DBA use was allergic to cosmetic glue, and the influence of immunological cross-reaction of acrylates was suggested.
Conclusion: We consider that DBA use is inadequate for wounds with an improper margin and in dry and low-skin barrier areas such as the trunk and limbs because it may induce irritant CD and sensitization of DBA and subsequent allergic CD. Frequent use can also induce sensitization. If patients have a history of acrylate allergies, DBA use should be avoided because immunological cross-reaction from acetylates could result.
Figures



Similar articles
-
Allergic contact dermatitis to Dermabond Prineo after abdominal wound closure for anterior lumbar interbody fusion: case report.J Spine Surg. 2023 Jun 30;9(2):191-194. doi: 10.21037/jss-22-93. Epub 2023 Jun 1. J Spine Surg. 2023. PMID: 37435324 Free PMC article.
-
Allergic contact dermatitis due to the liquid skin adhesive Dermabond® predominantly occurs after the first exposure.Contact Dermatitis. 2021 Feb;84(2):103-108. doi: 10.1111/cod.13700. Epub 2020 Sep 29. Contact Dermatitis. 2021. PMID: 32909284
-
Allergic Contact Dermatitis to Dermabond Prineo After Elective Orthopedic Surgery.Orthopedics. 2020 Nov 1;43(6):e515-e522. doi: 10.3928/01477447-20200827-01. Epub 2020 Sep 3. Orthopedics. 2020. PMID: 32882052
-
Contact Dermatitis and Medical Adhesives: A Review.Cureus. 2021 Mar 24;13(3):e14090. doi: 10.7759/cureus.14090. Cureus. 2021. PMID: 33903846 Free PMC article. Review.
-
[Sensitization to acrylates caused by artificial acrylic nails: review of 15 cases].Actas Dermosifiliogr. 2008 Dec;99(10):788-94. Actas Dermosifiliogr. 2008. PMID: 19091218 Review. Spanish.
Cited by
-
Silk Bioprotein as a Novel Surgical-Site Wound Dressing: A Prospective, Randomized, Single-Blinded, Superiority Clinical Trial.Aesthet Surg J Open Forum. 2023 Oct 20;5:ojad071. doi: 10.1093/asjof/ojad071. eCollection 2023. Aesthet Surg J Open Forum. 2023. PMID: 37899912 Free PMC article.
-
Adhesive Cements That Bond Soft Tissue Ex Vivo.Materials (Basel). 2019 Aug 3;12(15):2473. doi: 10.3390/ma12152473. Materials (Basel). 2019. PMID: 31382566 Free PMC article.
-
Effectiveness of securing central venous catheters with topical tissue adhesive in patients undergoing cardiac surgery: a randomized controlled pilot study.BMC Anesthesiol. 2021 Mar 8;21(1):70. doi: 10.1186/s12871-021-01282-0. BMC Anesthesiol. 2021. PMID: 33685394 Free PMC article. Clinical Trial.
-
Skin hypersensitivity following application of tissue adhesive (2-octyl cyanoacrylate).Proc (Bayl Univ Med Cent). 2021 Jun 15;34(6):736-738. doi: 10.1080/08998280.2021.1935140. eCollection 2021. Proc (Bayl Univ Med Cent). 2021. PMID: 34733007 Free PMC article.
-
Dermatologic Complications Following Cosmetic and Reconstructive Plastic Surgery: A Systematic Review of the Literature.Aesthetic Plast Surg. 2021 Dec;45(6):3005-3018. doi: 10.1007/s00266-021-02362-9. Epub 2021 Jul 6. Aesthetic Plast Surg. 2021. PMID: 34231016
References
-
- Singer AJ, Perry LC, Allen RL., Jr In vivo study of wound bursting strength and compliance of topical skin adhesives. Acad Emerg Med. 2008;15:1290. - PubMed
-
- Bhende S, Rothenburger S, Spangler DJ, et al. In vitro assessment of microbial barrier properties of Dermabond topical skin adhesive. Surg Infect (Larchmt). 2002;3:251. - PubMed
-
- Quinn J, Wells G, Sutcliffe T, et al. A randomized trial comparing octylcyanoacrylate tissue adhesive and sutures in the management of lacerations. JAMA. 1997;277:1527. - PubMed
-
- Maw JL, Quinn JV. Cyanoacrylate tissue adhesives. Amer J Cosmetic Surg. 1997;14:413.
-
- Miyzaki K, Sunaga A, Sugawara Y. Assessment of cleft lip scar closed with 2-octyl cyanoacrylate. J Jpn PRS. 2011;31:349.
LinkOut - more resources
Full Text Sources
