TRIM9 and TRIM67 Are New Targets in Paraneoplastic Cerebellar Degeneration
- PMID: 30350014
- PMCID: PMC6445697
- DOI: 10.1007/s12311-018-0987-5
TRIM9 and TRIM67 Are New Targets in Paraneoplastic Cerebellar Degeneration
Abstract
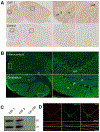
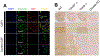
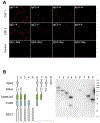
To describe autoantibodies (Abs) against tripartite motif-containing (TRIM) protein 9 and 67 in two patients with paraneoplastic cerebellar degeneration (PCD) associated with lung adenocarcinoma. Abs were characterized using immunohistochemistry, Western blotting, cultures of murine cortical, and hippocampal neurons, immunoprecipitation, mass spectrometry, knockout mice for Trim9 and 67, and cell-based assay. Control samples included sera from 63 patients with small cell lung cancer without any paraneoplastic neurological syndrome, 36 patients with lung adenocarcinoma and PNS, CSF from 100 patients with autoimmune encephalitis, and CSF from 165 patients with neurodegenerative diseases. We found Abs targeting TRIM9 and TRIM67 at high concentration in the serum and the cerebrospinal fluid (CSF) of a 78-year-old woman and a 65-year-old man. Both developed subacute severe cerebellar ataxia. Brain magnetic resonance imaging found no abnormality and no cerebellar atrophy. Both had CSF inflammation with mild pleiocytosis and a few oligoclonal bands. We identified a pulmonary adenocarcinoma, confirming the paraneoplastic neurological syndrome in both patients. They received immunomodulatory and cancer treatments without improvement of cerebellar ataxia, even though both were in remission of their cancer (for more than 10 years in one patient). Anti-TRIM9 and anti-TRIM67 Abs were specific to these two patients. All control serum and CSF samples tested were negative for anti-TRIM9 and 67. Anti-TRIM9 and anti-TRIM67 Abs appeared to be specific biomarkers of PCD and should be added to the panel of antigens tested when this is suspected.
Keywords: Autoantibodies; Lung cancer; Paraneoplastic cerebellar disorders; TRIM67; TRIM9.
Conflict of interest statement
Figures

References
-
- Honnorat J, Cartalat-Carel S. Advances in paraneoplastic neurological syndromes. Curr Opin Oncol. 2004;16(6):614–620. - PubMed
-
- Greenlee JE, Brashear HR. Antibodies to cerebellar Purkinje cells in patients with paraneoplastic cerebellar degeneration and ovarian carcinoma. Ann Neurol 1983; 14(6): 609–13. - PubMed
-
- Ducray F, Demarquay G, Graus F, Decullier E, Antoine JC, Giometto B, Psimaras D, Delattre JY, Carpentier AF, Honnorat J. Seronegative paraneoplastic cerebellar degeneration: the PNS Euronetwork experience. Eur J Neurol. 2014. May;21(5):731–5. - PubMed

