Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers
- PMID: 30350585
- PMCID: PMC6516866
- DOI: 10.1021/acs.chemrev.8b00363
Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers
Abstract
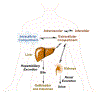
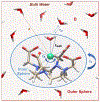
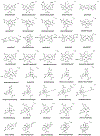
Tens of millions of contrast-enhanced magnetic resonance imaging (MRI) exams are performed annually around the world. The contrast agents, which improve diagnostic accuracy, are almost exclusively small, hydrophilic gadolinium(III) based chelates. In recent years concerns have arisen surrounding the long-term safety of these compounds, and this has spurred research into alternatives. There has also been a push to develop new molecularly targeted contrast agents or agents that can sense pathological changes in the local environment. This comprehensive review describes the state of the art of clinically approved contrast agents, their mechanism of action, and factors influencing their safety. From there we describe different mechanisms of generating MR image contrast such as relaxation, chemical exchange saturation transfer, and direct detection and the types of molecules that are effective for these purposes. Next we describe efforts to make safer contrast agents either by increasing relaxivity, increasing resistance to metal ion release, or by moving to gadolinium(III)-free alternatives. Finally we survey approaches to make contrast agents more specific for pathology either by direct biochemical targeting or by the design of responsive or activatable contrast agents.
Figures


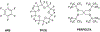











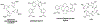
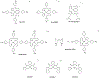



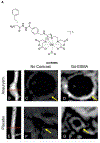
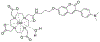
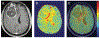


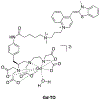















References
-
- Runge VM Critical Questions Regarding Gadolinium Deposition in the Brain and Body after Injections of the Gadolinium-Based Contrast Agents, Safety, and Clinical Recommendations in Consideration of the EMA’s Pharmacovigilance and Risk Assessment Committee Recommendation for Suspension of the Marketing Authorizations for 4 Linear Agents. Invest. Radiol 2017, 52, 317–323. - PubMed
-
- Caravan P; Ellison JJ; McMurry TJ; Lauffer RB Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev 1999, 99, 2293–2352. - PubMed
-
- Edelman RR; Hesselink JR; Zlatkin MB; Crues JV III Clinical Magnetic Resonance Imaging - Volume 3 Elsevier Health: St. Louis, 2006.
-
- Prince MR; Zhang HL; Zou ZT; Staron RB; Brill PW Incidence of Immediate Gadolinium Contrast Media Reactions. Am. J. Roentgenol 2011, 196, W138–W143. - PubMed
-
- Grobner T Gadolinium – A Specific Trigger for the Development of Nephrogenic Fibrosing Dermopathy and Nephrogenic Systemic Fibrosis? Nephrol. Dial. Transplant 2006, 21, 1104–1108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

