Decoupling the Functional Roles of Cationic and Hydrophobic Groups in the Antimicrobial and Hemolytic Activities of Methacrylate Random Copolymers
- PMID: 30350596
- PMCID: PMC6238640
- DOI: 10.1021/acs.biomac.8b01256
Decoupling the Functional Roles of Cationic and Hydrophobic Groups in the Antimicrobial and Hemolytic Activities of Methacrylate Random Copolymers
Abstract
In this study, we report the antimicrobial and hemolytic activities of ternary statistical methacrylate copolymers consisting of cationic ammonium (amino-ethyl methacrylate: AEMA), hydrophobic alkyl (ethyl methacrylate: EMA), and neutral hydroxyl (hydroxyethyl methacrylate: HEMA) side chain monomers. The cationic and hydrophobic functionalities of copolymers mimic the cationic amphiphilicity of naturally occurring antimicrobial peptides (AMPs). The HEMA monomer units were used to separately modulate the compositions of cationic and hydrophobic monomers, and we investigated the effect of each component on the antimicrobial and hemolytic activities of copolymers. Our data indicated that increasing the number of cationic groups of the copolymers to be more than 30 mol % did not increase their antimicrobial activity against Escherichia coli. The number of cationic side chains in a polymer chain at this threshold is 5.5-7.7, which is comparable to those of natural antimicrobial peptides such as maginin (+6). The MIC values of copolymers with >30 mol % of AEMA depend on only the mol % of EMA, indicating that the hydrophobic interactions of the copolymers with E. coli cell membranes determine the antimicrobial activity of copolymers. These results suggest that the roles of cationic and hydrophobic groups can be controlled independently by design in the ternary copolymers studied here.
Figures


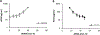

References
-
- Kenawy ER; Worley SD; Broughton R, Biomacromolecules 2007, 8 (5), 1359–1384. - PubMed
-
- Timofeeva L; Kleshcheva N, Appl. Microbiol. Biotechnol 2011, 89 (3), 475–492. - PubMed
-
- Engler AC; Wiradharma N; Ong ZY; Coady DJ; Hedrick JL; Yang YY, Nano Today 2012, 7 (3), 201–222.
-
- Ganewatta MS; Tang CB, Polymer 2015, 63, A1–A29.
-
- Krumm C; Tiller JC, Chapter 15 Antimicrobial Polymers and Surfaces - Natural Mimics or Surpassing Nature? In Bio-inspired Polymers, The Royal Society of Chemistry: 2017; pp 490–522.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

