NR5A1 gene variants repress the ovarian-specific WNT signaling pathway in 46,XX disorders of sex development patients
- PMID: 30350900
- PMCID: PMC6492147
- DOI: 10.1002/humu.23672
NR5A1 gene variants repress the ovarian-specific WNT signaling pathway in 46,XX disorders of sex development patients
Abstract
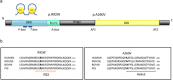
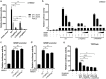
Several recent reports have described a missense variant in the gene NR5A1 (c.274C>T; p.Arg92Trp) in a significant number of 46,XX ovotesticular or testicular disorders of sex development (DSDs) cases. The affected residue falls within the DNA-binding domain of the NR5A1 protein, however the exact mechanism by which it causes testicular development in 46,XX individuals remains unclear. We have screened a cohort of 26 patients with 46,XX (ovo)testicular DSD and identified three unrelated individuals with this NR5A1 variant (p.Arg92Trp), as well as one patient with a novel NR5A1 variant (c.779C>T; p.Ala260Val). We examined the functional effect of these changes, finding that while protein levels and localization were unaffected, variant NR5A1 proteins repress the WNT signaling pathway and have less ability to upregulate the anti-testis gene NR0B1. These findings highlight how NR5A1 variants impact ovarian differentiation across multiple pathways, resulting in a switch from ovarian to testis development in genetic females.
Keywords: 46,XX ovotesticular DSD; 46,XX testicular DSD; NR5A1; SF1; disorders of sex development.
© 2018 The Authors. Human Mutation published by Wiley Periodicals, Inc.
Figures

References
-
- Baetens, D , Stoop, H. , Peelman, F. , Todeschini, A.‐L. , Rosseel, T. , Coppieters, F. , … Cools, M. (2017). NR5A1 is a novel disease gene for 46,XX testicular and ovotesticular disorders of sex development. Genetics in Medicine: Official Journal of the American College of Medical Genetics, 19(4), 367–376. 10.1038/gim.2016.118 - DOI - PMC - PubMed
-
- Bashamboo, A. , Donohoue, P. A. , Vilain, E. , Rojo, S. , Calvel, P. , Seneviratne, S. N. , … Achermann, J. C. (2016). A recurrent p.Arg92Trp variant in steroidogenic factor‐1 (NR5A1) can act as a molecular switch in human sex development. Human Molecular Genetics, 25(16), 3446–3453. 10.1093/hmg/ddw186 - DOI - PMC - PubMed
-
- Bashamboo, A. , Eozenou, C. , Jorgensen, A. , Bignon‐Topalovic, J. , Siffroi, J. P. , Hyon, C. , … McElreavey, K. (2018). Loss of Function of the Nuclear Receptor NR2F2, Encoding COUP‐TF2, Causes Testis Development and Cardiac Defects in 46,XX Children. American Journal of Human Genetics, 102(3), 487–493. 10.1016/j.ajhg.2018.01.021 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

