Atrophy subtypes in prodromal Alzheimer's disease are associated with cognitive decline
- PMID: 30351346
- PMCID: PMC6669409
- DOI: 10.1093/brain/awy264
Atrophy subtypes in prodromal Alzheimer's disease are associated with cognitive decline
Abstract
Alzheimer's disease is a heterogeneous disorder. Understanding the biological basis for this heterogeneity is key for developing personalized medicine. We identified atrophy subtypes in Alzheimer's disease dementia and tested whether these subtypes are already present in prodromal Alzheimer's disease and could explain interindividual differences in cognitive decline. First we retrospectively identified atrophy subtypes from structural MRI with a data-driven cluster analysis in three datasets of patients with Alzheimer's disease dementia: discovery data (dataset 1: n = 299, age = 67 ± 8, 50% female), and two independent external validation datasets (dataset 2: n = 181, age = 66 ± 7, 52% female; dataset 3: n = 227, age = 74 ± 8, 44% female). Subtypes were compared on clinical, cognitive and biological characteristics. Next, we classified prodromal Alzheimer's disease participants (n = 603, age = 72 ± 8, 43% female) according to the best matching subtype to their atrophy pattern, and we tested whether subtypes showed cognitive decline in specific domains. In all Alzheimer's disease dementia datasets we consistently identified four atrophy subtypes: (i) medial-temporal predominant atrophy with worst memory and language function, older age, lowest CSF tau levels and highest amount of vascular lesions; (ii) parieto-occipital atrophy with poor executive/attention and visuospatial functioning and high CSF tau; (iii) mild atrophy with best cognitive performance, young age, but highest CSF tau levels; and (iv) diffuse cortical atrophy with intermediate clinical, cognitive and biological features. Prodromal Alzheimer's disease participants classified into one of these subtypes showed similar subtype characteristics at baseline as Alzheimer's disease dementia subtypes. Compared across subtypes in prodromal Alzheimer's disease, the medial-temporal subtype showed fastest decline in memory and language over time; the parieto-occipital subtype declined fastest on executive/attention domain; the diffuse subtype in visuospatial functioning; and the mild subtype showed intermediate decline in all domains. Robust atrophy subtypes exist in Alzheimer's disease with distinct clinical and biological disease expression. Here we observe that these subtypes can already be detected in prodromal Alzheimer's disease, and that these may inform on expected trajectories of cognitive decline.
Figures


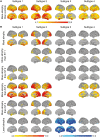
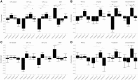
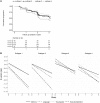
Comment in
-
Exploring Alzheimer's disease subtypes at the prodromal stage.Brain. 2018 Dec 1;141(12):3285-3287. doi: 10.1093/brain/awy282. Brain. 2018. PMID: 30496363 No abstract available.
References
-
- Blennow K, Mattsson N, Schöll M, Hansson O, Zetterberg H. Amyloid biomarkers in Alzheimer’s disease. Trends Pharmacol Sci 2015; 36: 297–309. - PubMed
-
- Dice L. Measures of the amount of ecologic association between species. Ecology 1945; 26: 297–302.
-
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol 2014; 13: 614–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

