Potential Role for Urine Polymerase Chain Reaction in the Diagnosis of Whipple's Disease
- PMID: 30351371
- PMCID: PMC6424077
- DOI: 10.1093/cid/ciy664
Potential Role for Urine Polymerase Chain Reaction in the Diagnosis of Whipple's Disease
Abstract
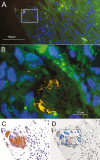
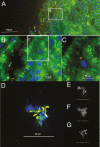
Background: Whipple's disease (WD) is a rare infection with Tropheryma whipplei that is fatal if untreated. Diagnosis is challenging and currently based on invasive sampling. In a case of WD diagnosed from a kidney biopsy, we observed morphologically-intact bacteria within the glomerular capsular space and tubular lumens. This raised the questions of whether renal filtration of bacteria is common in WD and whether polymerase chain reaction (PCR) testing of urine might serve as a diagnostic test for WD.
Methods: We prospectively investigated urine samples of 12 newly-diagnosed and 31 treated WD patients by PCR. As controls, we investigated samples from 110 healthy volunteers and patients with excluded WD or acute gastroenteritis.
Results: Out of 12 urine samples from independent, therapy-naive WD patients, 9 were positive for T. whipplei PCR. In 3 patients, fluorescence in situ hybridization visualized T. whipplei in urine. All control samples were negative, including those of 11 healthy carriers with T. whipplei-positive stool samples. In our study, the detection of T. whipplei in the urine of untreated patients correlated in all cases with WD.
Conclusions: T. whipplei is detectable by PCR in the urine of the majority of therapy-naive WD patients. With a low prevalence but far-reaching consequences upon diagnosis, invasive sampling for WD is mandatory and must be based on a strong suspicion. Urine testing could prevent patients from being undiagnosed for years. Urine may serve as a novel, easy-to-obtain specimen for guiding the initial diagnosis of WD, in particular in patients with extra-intestinal WD.
Keywords: Tropheryma whipplei; Whipple’s disease; electron microscopy; fluorescence in situ hybridization; real-time PCR.
© The Author(s) 2018. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures


Comment in
-
From Whipple Disease to Tropheryma whipplei Infection.Clin Infect Dis. 2019 Mar 19;68(7):1098-1099. doi: 10.1093/cid/ciy668. Clin Infect Dis. 2019. PMID: 30351351 No abstract available.
-
Potential Role for Urine Polymerase Chain Reaction in the Diagnosis of Whipple Disease.Clin Infect Dis. 2019 Aug 16;69(5):904-905. doi: 10.1093/cid/ciz094. Clin Infect Dis. 2019. PMID: 30945731 No abstract available.
-
Reply to Tison and Saraux.Clin Infect Dis. 2019 Aug 16;69(5):905. doi: 10.1093/cid/ciz095. Clin Infect Dis. 2019. PMID: 30945746 No abstract available.
References
-
- Schneider T, Moos V, Loddenkemper C, Marth T, Fenollar F, Raoult D. Whipple’s disease: new aspects of pathogenesis and treatment. Lancet Infect Dis 2008; 8:179–90. - PubMed
-
- La Scola B, Fenollar F, Fournier PE, Altwegg M, Mallet MN, Raoult D. Description of Tropheryma whipplei gen. nov., sp. nov., the Whipple’s disease bacillus. Int J Syst Evol Microbiol 2001; 51:1471–9. - PubMed
-
- Lagier JC, Cammilleri S, Raoult D. Classic Whipple’s disease diagnosed by (18)F-fluorodeoxyglucose PET. Lancet Infect Dis 2016; 16:130. - PubMed
-
- Angelakis E, Fenollar F, Lepidi H, Birg ML, Raoult D. Tropheryma whipplei in the skin of patients with classic Whipple’s disease. J Infect 2010; 61:266–9. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

