Regional diastolic dysfunction in post-infarction heart failure: role of local mechanical load and SERCA expression
- PMID: 30351410
- PMCID: PMC6432054
- DOI: 10.1093/cvr/cvy257
Regional diastolic dysfunction in post-infarction heart failure: role of local mechanical load and SERCA expression
Abstract
Aims: Regional heterogeneities in contraction contribute to heart failure with reduced ejection fraction (HFrEF). We aimed to determine whether regional changes in myocardial relaxation similarly contribute to diastolic dysfunction in post-infarction HFrEF, and to elucidate the underlying mechanisms.
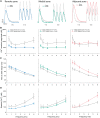
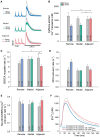
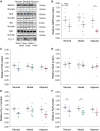
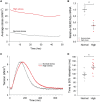
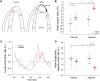
Methods and results: Using the magnetic resonance imaging phase-contrast technique, we examined local diastolic function in a rat model of post-infarction HFrEF. In comparison with sham-operated animals, post-infarction HFrEF rats exhibited reduced diastolic strain rate adjacent to the scar, but not in remote regions of the myocardium. Removal of Ca2+ within cardiomyocytes governs relaxation, and we indeed found that Ca2+ transients declined more slowly in cells isolated from the adjacent region. Resting Ca2+ levels in adjacent zone myocytes were also markedly elevated at high pacing rates. Impaired Ca2+ removal was attributed to a reduced rate of Ca2+ sequestration into the sarcoplasmic reticulum (SR), due to decreased local expression of the SR Ca2+ ATPase (SERCA). Wall stress was elevated in the adjacent region. Using ex vivo experiments with loaded papillary muscles, we demonstrated that high mechanical stress is directly linked to SERCA down-regulation and slowing of relaxation. Finally, we confirmed that regional diastolic dysfunction is also present in human HFrEF patients. Using echocardiographic speckle-tracking of patients enrolled in the LEAF trial, we found that in comparison with controls, post-infarction HFrEF subjects exhibited reduced diastolic train rate adjacent to the scar, but not in remote regions of the myocardium.
Conclusion: Our data indicate that relaxation varies across the heart in post-infarction HFrEF. Regional diastolic dysfunction in this condition is linked to elevated wall stress adjacent to the infarction, resulting in down-regulation of SERCA, disrupted diastolic Ca2+ handling, and local slowing of relaxation.
Keywords: Cardiomyocyte calcium cycling; Diastolic dysfunction; Heart failure; Post-infarction remodelling; Wall stress.
© The Author(s) 2018. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

Comment in
-
SERCA in heterogeneity of diastolic dysfunction in post-infarction heart failure with reduced ejection fraction.Cardiovasc Res. 2019 Mar 15;115(4):693-695. doi: 10.1093/cvr/cvz017. Cardiovasc Res. 2019. PMID: 30715200 No abstract available.
References
-
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016;37:2129–2200. - PubMed
-
- Kramer CM, Lima JA, Reichek N, Ferrari VA, Llaneras MR, Palmon LC, Yeh IT, Tallant B, Axel L.. Regional differences in function within noninfarcted myocardium during left ventricular remodeling. Circulation 1993;88:1279–1288. - PubMed
-
- Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL.. Systolic and diastolic heart failure in the community. JAMA 2006;296:2209–2216. - PubMed
-
- Brucks S, Little WC, Chao T, Kitzman DW, Wesley-Farrington D, Gandhi S, Shihabi ZK.. Contribution of left ventricular diastolic dysfunction to heart failure regardless of ejection fraction. Am J Cardiol 2005;95:603–606. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

