Size-Selective Hydroformylation by a Rhodium Catalyst Confined in a Supramolecular Cage
- PMID: 30351486
- PMCID: PMC6391983
- DOI: 10.1002/chem.201804333
Size-Selective Hydroformylation by a Rhodium Catalyst Confined in a Supramolecular Cage
Abstract
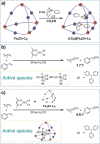
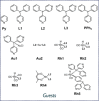
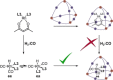
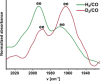
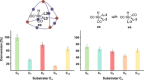
Size-selective hydroformylation of terminal alkenes was attained upon embedding a rhodium bisphosphine complex in a supramolecular metal-organic cage that was formed by subcomponent self-assembly. The catalyst was bound in the cage by a ligand-template approach, in which pyridyl-zinc(II) porphyrin interactions led to high association constants (>105 m-1 ) for the binding of the ligands and the corresponding rhodium complex. DFT calculations confirm that the second coordination sphere forces the encapsulated active species to adopt the ee coordination geometry (i.e., both phosphine ligands in equatorial positions), in line with in situ high-pressure IR studies of the host-guest complex. The window aperture of the cage decreases slightly upon binding the catalyst. As a result, the diffusion of larger substrates into the cage is slower compared to that of smaller substrates. Consequently, the encapsulated rhodium catalyst displays substrate selectivity, converting smaller substrates faster to the corresponding aldehydes. This selectivity bears a resemblance to an effect observed in nature, where enzymes are able to discriminate between substrates based on shape and size by embedding the active site deep inside the hydrophobic pocket of a bulky protein structure.
Keywords: cage compounds; hydroformylation; porphyrins; substrate selectivity; supramolecular chemistry.
© 2018 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Tuning the Porphyrin Building Block in Self-Assembled Cages for Branched-Selective Hydroformylation of Propene.Chemistry. 2017 Oct 20;23(59):14769-14777. doi: 10.1002/chem.201702113. Epub 2017 Aug 17. Chemistry. 2017. PMID: 28608592 Free PMC article.
-
Ligand Template Strategies for Catalyst Encapsulation.Acc Chem Res. 2018 Sep 18;51(9):2115-2128. doi: 10.1021/acs.accounts.8b00345. Epub 2018 Aug 23. Acc Chem Res. 2018. PMID: 30137959 Free PMC article.
-
Enantioselective hydroformylation by a Rh-catalyst entrapped in a supramolecular metallocage.J Am Chem Soc. 2015 Feb 25;137(7):2680-7. doi: 10.1021/ja512637k. Epub 2015 Feb 11. J Am Chem Soc. 2015. PMID: 25632976
-
Palladium-catalyzed Reppe carbonylation.Chem Rev. 2001 Nov;101(11):3435-56. doi: 10.1021/cr010328q. Chem Rev. 2001. PMID: 11840990 Review.
-
Transition metal catalysis in confined spaces.Chem Soc Rev. 2015 Jan 21;44(2):433-48. doi: 10.1039/c4cs00192c. Chem Soc Rev. 2015. PMID: 25340992 Review.
Cited by
-
Recent developments in the construction and applications of platinum-based metallacycles and metallacages via coordination.Chem Soc Rev. 2020 Jun 21;49(12):3889-3919. doi: 10.1039/d0cs00038h. Epub 2020 May 15. Chem Soc Rev. 2020. PMID: 32412574 Free PMC article.
-
How to not build a cage: endohedral functionalization of polyoxometalate-based metal-organic polyhedra.Chem Sci. 2021 Apr 3;12(21):7361-7368. doi: 10.1039/d1sc01243f. Chem Sci. 2021. PMID: 34163825 Free PMC article.
-
Beyond symmetric self-assembly and effective molarity: unlocking functional enzyme mimics with robust organic cages.Beilstein J Org Chem. 2025 Feb 24;21:421-443. doi: 10.3762/bjoc.21.30. eCollection 2025. Beilstein J Org Chem. 2025. PMID: 40041197 Free PMC article.
-
Substrate scope driven optimization of an encapsulated hydroformylation catalyst.Catal Sci Technol. 2024 Feb 16;14(7):1837-1847. doi: 10.1039/d4cy00051j. eCollection 2024 Apr 2. Catal Sci Technol. 2024. PMID: 38571547 Free PMC article.
-
Metallocavitins as Promising Industrial Catalysts: Recent Advances.Front Chem. 2022 Feb 11;9:806800. doi: 10.3389/fchem.2021.806800. eCollection 2021. Front Chem. 2022. PMID: 35223777 Free PMC article. Review.
References
-
- None
-
- Seechurn C. C. C. J., Kitching M. O., Colacot T. J., Snieckus V., Angew. Chem. Int. Ed. 2012, 51, 5062–5085; - PubMed
- Angew. Chem. 2012, 124, 5150–5174;
-
- Noyori R., Nat. Chem. 2009, 1, 5–6; - PubMed
-
- Franke R., Selent D., Börner A., Chem. Rev. 2012, 112, 5675–5732; - PubMed
-
- Cornils B., Herrmann W. A., Applied Homogeneous Catalysis with Organometallic Compounds, Wiley-VCH, Weinheim, 1996;
LinkOut - more resources
Full Text Sources
Research Materials

