Evaluation of strain coverage of the multicomponent meningococcal serogroup B vaccine (4CMenB) administered in infants according to different immunisation schedules
- PMID: 30352000
- PMCID: PMC6605712
- DOI: 10.1080/21645515.2018.1537756
Evaluation of strain coverage of the multicomponent meningococcal serogroup B vaccine (4CMenB) administered in infants according to different immunisation schedules
Abstract
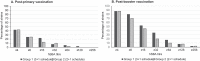
The 4-component vaccine 4CMenB, developed against invasive disease caused by meningococcal serogroup B, is approved for use in infants in several countries worldwide. 4CMenB is mostly used as 3 + 1 schedule, except for the UK, where a 2 + 1 schedule is used, and where the vaccine showed an effectiveness of 82.9%. Here we compared the coverage of two 4CMenB vaccination schedules (3 + 1 [2.5, 3.5, 5, 11 months] versus 2 + 1 [3.5, 5, 11 months of age]) against 40 serogroup B strains, representative of epidemiologically-relevant isolates circulating in England and Wales in 2007-2008, using sera from a previous phase 3b clinical trial. The strains were tested using hSBA on pooled sera of infants, collected at one month post-primary and booster vaccination. 4CMenB coverage was defined as the percentage of strains with positive killing (hSBA titres ≥ 4 after immunisation and negative baseline hSBA titres < 2). Coverage of 4CMenB was 40.0% (95% confidence interval [CI]: 24.9-56.7) and 87.5% (95%CI: 73.2-95.8) at one month post-primary and booster vaccination, respectively, regardless of immunisation schedule. Using a more conservative threshold (post-immunisation hSBA titres ≥ 8; baseline ≤ 2), at one month post-booster dose, strain coverages were 80% (3 + 1) and 70% (2 + 1). We used a linear regression model to assess correlation between post-immunisation hSBA data for each strain in the two groups; Pearson's correlation coefficients were 0.93 and 0.99 at one month post-primary and booster vaccination. Overall, there is no evidence for a difference in strain coverage when 4CMenB is administered according to a 3 + 1 or 2 + 1 infant vaccination schedule.
Keywords: 4CMenB vaccine; infant immunisation schedule; meningococcal serogroup B; pooled sera; serum bactericidal antibody assay; strain coverage.
Figures




Similar articles
-
4CMenB journey to the 10-year anniversary and beyond.Hum Vaccin Immunother. 2024 Dec 31;20(1):2357924. doi: 10.1080/21645515.2024.2357924. Epub 2024 Jul 8. Hum Vaccin Immunother. 2024. PMID: 38976659 Free PMC article. Review.
-
Breadth of immune response, immunogenicity, reactogenicity, and safety for a pentavalent meningococcal ABCWY vaccine in healthy adolescents and young adults: results from a phase 3, randomised, controlled observer-blinded trial.Lancet Infect Dis. 2025 May;25(5):560-573. doi: 10.1016/S1473-3099(24)00667-4. Epub 2024 Dec 5. Lancet Infect Dis. 2025. PMID: 39647494 Clinical Trial.
-
Bactericidal antibody against a representative epidemiological meningococcal serogroup B panel confirms that MATS underestimates 4CMenB vaccine strain coverage.Vaccine. 2013 Oct 9;31(43):4968-74. doi: 10.1016/j.vaccine.2013.08.006. Epub 2013 Aug 14. Vaccine. 2013. PMID: 23954380
-
Persistence of bactericidal antibodies following early infant vaccination with a serogroup B meningococcal vaccine and immunogenicity of a preschool booster dose.CMAJ. 2013 Oct 15;185(15):E715-24. doi: 10.1503/cmaj.130257. Epub 2013 Sep 23. CMAJ. 2013. PMID: 24062178 Free PMC article. Clinical Trial.
-
Multicomponent meningococcal serogroup B vaccine (4CMenB; Bexsero(®)): a review of its use in primary and booster vaccination.BioDrugs. 2013 Jun;27(3):263-74. doi: 10.1007/s40259-013-0029-2. BioDrugs. 2013. PMID: 23575646 Review.
Cited by
-
4CMenB journey to the 10-year anniversary and beyond.Hum Vaccin Immunother. 2024 Dec 31;20(1):2357924. doi: 10.1080/21645515.2024.2357924. Epub 2024 Jul 8. Hum Vaccin Immunother. 2024. PMID: 38976659 Free PMC article. Review.
-
First immunogenicity data for the UK serogroup B meningococcal vaccination schedule in infants.Lancet Infect Dis. 2021 May;21(5):586-587. doi: 10.1016/S1473-3099(20)30690-3. Epub 2021 Jan 8. Lancet Infect Dis. 2021. PMID: 33428869 Free PMC article. No abstract available.
-
Exploring the Ability of Meningococcal Vaccines to Elicit Mucosal Immunity: Insights from Humans and Mice.Pathogens. 2021 Jul 18;10(7):906. doi: 10.3390/pathogens10070906. Pathogens. 2021. PMID: 34358056 Free PMC article. Review.
-
Effectiveness and Impact of the 4CMenB Vaccine against Group B Meningococcal Disease in Two Italian Regions Using Different Vaccination Schedules: A Five-Year Retrospective Observational Study (2014-2018).Vaccines (Basel). 2020 Aug 22;8(3):469. doi: 10.3390/vaccines8030469. Vaccines (Basel). 2020. PMID: 32842669 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
