Muscarinic receptors regulate auditory and prefrontal cortical communication during auditory processing
- PMID: 30352212
- PMCID: PMC6400225
- DOI: 10.1016/j.neuropharm.2018.10.027
Muscarinic receptors regulate auditory and prefrontal cortical communication during auditory processing
Abstract
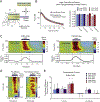
Much of our understanding about how acetylcholine modulates prefrontal cortical (PFC) networks comes from behavioral experiments that examine cortical dynamics during highly attentive states. However, much less is known about how PFC is recruited during passive sensory processing and how acetylcholine may regulate connectivity between cortical areas outside of task performance. To investigate the involvement of PFC and cholinergic neuromodulation in passive auditory processing, we performed simultaneous recordings in the auditory cortex (AC) and PFC in awake head fixed mice presented with a white noise auditory stimulus in the presence or absence of local cholinergic antagonists in AC. We found that a subset of PFC neurons were strongly driven by auditory stimuli even when the stimulus had no associative meaning, suggesting PFC monitors stimuli under passive conditions. We also found that cholinergic signaling in AC shapes the strength of auditory driven responses in PFC, by modulating the intra-cortical sensory response through muscarinic interactions in AC. Taken together, these findings provide novel evidence that cholinergic mechanisms have a continuous role in cortical gating through muscarinic receptors during passive processing and expand traditional views of prefrontal cortical function and the contributions of cholinergic modulation in cortical communication.
Keywords: Acetylcholine; Auditory cortex; Cortical gating; Prefrontal cortex; Sensory processing.
Copyright © 2018. Published by Elsevier Ltd.
Figures




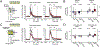

Similar articles
-
Tone identification behavior in Rattus norvegicus: muscarinic receptor blockage lowers responsiveness in nontarget selective neurons, while nicotinic receptor blockage selectively lowers target responses.Eur J Neurosci. 2017 Jul;46(2):1779-1789. doi: 10.1111/ejn.13611. Epub 2017 Jul 7. Eur J Neurosci. 2017. PMID: 28544049
-
Muscarinic dependence of nucleus basalis induced conditioned receptive field plasticity.Neuroreport. 2001 May 25;12(7):1537-42. doi: 10.1097/00001756-200105250-00047. Neuroreport. 2001. PMID: 11388444
-
Model-based prediction of muscarinic receptor function from auditory mismatch negativity responses.Neuroimage. 2021 Aug 15;237:118096. doi: 10.1016/j.neuroimage.2021.118096. Epub 2021 May 1. Neuroimage. 2021. PMID: 33940149
-
Neuromodulation of Persistent Activity and Working Memory Circuitry in Primate Prefrontal Cortex by Muscarinic Receptors.Front Neural Circuits. 2021 Mar 15;15:648624. doi: 10.3389/fncir.2021.648624. eCollection 2021. Front Neural Circuits. 2021. PMID: 33790746 Free PMC article. Review.
-
Evolution in Neuromodulation-The Differential Roles of Acetylcholine in Higher Order Association vs. Primary Visual Cortices.Front Neural Circuits. 2018 Aug 28;12:67. doi: 10.3389/fncir.2018.00067. eCollection 2018. Front Neural Circuits. 2018. PMID: 30210306 Free PMC article. Review.
Cited by
-
Neuromodulation in circuits of aversive emotional learning.Nat Neurosci. 2019 Oct;22(10):1586-1597. doi: 10.1038/s41593-019-0503-3. Epub 2019 Sep 24. Nat Neurosci. 2019. PMID: 31551602 Review.
-
Activation of M1 cholinergic receptors in mouse somatosensory cortex enhances information processing and detection behaviour.Commun Biol. 2024 Jan 2;7(1):3. doi: 10.1038/s42003-023-05699-w. Commun Biol. 2024. PMID: 38168628 Free PMC article.
-
The neural circuit mechanism for auditory responses in the mediodorsal thalamic nucleus of awake mice.Commun Biol. 2025 Jun 6;8(1):884. doi: 10.1038/s42003-025-08329-9. Commun Biol. 2025. PMID: 40481219 Free PMC article.
-
Distinct Spiking Patterns of Excitatory and Inhibitory Neurons and LFP Oscillations in Prefrontal Cortex During Sensory Discrimination.Front Physiol. 2021 Feb 11;12:618307. doi: 10.3389/fphys.2021.618307. eCollection 2021. Front Physiol. 2021. PMID: 33679434 Free PMC article.
-
Parvalbumin neurons enhance temporal coding and reduce cortical noise in complex auditory scenes.Commun Biol. 2023 Jul 19;6(1):751. doi: 10.1038/s42003-023-05126-0. Commun Biol. 2023. PMID: 37468561 Free PMC article.
References
-
- Ashe JH, McKenna TM, Weinberger NM, 1989. Cholinergic modulationoffrequency receptive fields in auditory cortex: II. Frequency-specific effects of anticholinesterases provide evidence for a modulatory action of endogenous ACh. Synapse 4, 44–54. - PubMed
-
- Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW, 2001. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cerebr. Cortex 11, 441–451. - PubMed
-
- Bandrowski AE, Moore SL, Ashe JH, 2001. Cholinergic synaptic potentials in the supragranular layers of auditory cortex. Synapse 41, 118–130. - PubMed
-
- Barak S, 2009. Modeling cholinergic aspects of schizophrenia: focus on the anti-muscarinic syndrome. Behav. Brain Res. 204, 335–351. - PubMed
-
- Barbas H, 2007. Specialized elements of orbitofrontal cortex in primates. Ann. N. Y. Acad. Sci. 1121, 10–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

