Novel SLCO2A1 mutations cause gender-differentiated pachydermoperiostosis
- PMID: 30352415
- PMCID: PMC6223238
- DOI: 10.1530/EC-18-0326
Novel SLCO2A1 mutations cause gender-differentiated pachydermoperiostosis
Abstract
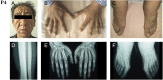
Primary hypertrophic osteoarthropathy (PHO) is a rare familial disorder with reduced penetrance for females. The genetic mutations associated with PHO have been identified in HPGD and SLCO2A1, which involved in prostaglandin E2 metabolism. Here, we report 5 PHO patients from four non-consanguineous families. Two heterozygous mutations in solute carrier organic anion transporter family member 2A1 (SLCO2A1) were identified in two brothers by whole-exome sequencing. Three heterozygous mutations and one homozygous mutation were identified in other three PHO families by Sanger sequencing. However, there was no mutation in HPGD. These findings confirmed that homozygous or compound heterozygous mutations of SLCO2A1 were the pathogenic cause of PHO. A female individual shared the same mutations in SLCO2A1 with her PHO brother but did not have any typical PHO symptoms. The influence of sex hormones on the pathogenesis of PHO and its implication were discussed.
Keywords: exome sequencing; female atypical phenotype; hormone therapeutics; primary hypertrophic osteoarthropathy; solute carrier organic anion transporter family member 2A1.
Figures




References
LinkOut - more resources
Full Text Sources

