Evaluation of bone marrow-derived mesenchymal stem cell quality from patients with congenital pseudoarthrosis of the tibia
- PMID: 30352605
- PMCID: PMC6199809
- DOI: 10.1186/s13018-018-0977-9
Evaluation of bone marrow-derived mesenchymal stem cell quality from patients with congenital pseudoarthrosis of the tibia
Abstract
Background: The treatment of congenital pseudoarthrosis of the tibia (CPT) remains challenging in pediatric orthopedics due to the difficulties in bone union, continuous angulation, joint stiffness, and severe limb length discrepancy. Mesenchymal stem cells (MSCs) therapy offers a complementary approach to improve the conventional surgical treatments. Although the autologous MSC treatment shows a promising strategy to promote bone healing in CPT patients, the quality of MSCs from CPT patients has not been well studied. The purpose of this study is to investigate the quality of MSCs isolated from patients with CPT.
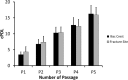
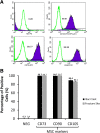
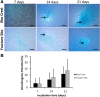
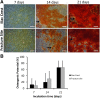
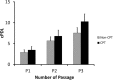
Methods: The bone marrow-derived MSCs from the fracture site and iliac crest of six CPT patients were isolated and compared. The cumulative population doubling level (cPDL), phenotype characteristics, and trilineage differentiation potency were observed to assess the quality of both MSCs.
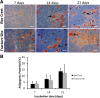
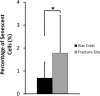
Results: There were no significant differences of the MSCs derived from the fracture site and the MSCs from the iliac crest of the subjects, in terms of cPDL, phenotype characteristics, and trilineage differentiation potency (all p > 0.05). However, MSCs from the fracture site had a higher senescence tendency than those from the iliac crest.
Conclusion: MSC quality is not the main reason for delayed bone regeneration in those with CPT. Thus, autologous MSC is a promising source for treating CPT patients.
Keywords: Cell differentiation; Mesenchymal stem cells; Osteocytes; Pseudoarthrosis.
Conflict of interest statement
Ethics approval and consent to participate
All participants were given an informed consent prior to the bone marrow aspiration. The protocols of this study were approved by the Ethics Committee of the Faculty of Medicine, Universitas Indonesia–Dr. Cipto Mangunkusumo General Hospital, and the studies were conducted in compliance with the Helsinki Declaration.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Abramowicz A, Gos M. Neurofibromin in neurofibromatosis type 1 mutations in NF1 gene as a cause of disease. Dev Period Med. 2014;18(3):297–306. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

