Species-level bacterial community profiling of the healthy sinonasal microbiome using Pacific Biosciences sequencing of full-length 16S rRNA genes
- PMID: 30352611
- PMCID: PMC6199724
- DOI: 10.1186/s40168-018-0569-2
Species-level bacterial community profiling of the healthy sinonasal microbiome using Pacific Biosciences sequencing of full-length 16S rRNA genes
Abstract
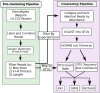
Background: Pan-bacterial 16S rRNA microbiome surveys performed with massively parallel DNA sequencing technologies have transformed community microbiological studies. Current 16S profiling methods, however, fail to provide sufficient taxonomic resolution and accuracy to adequately perform species-level associative studies for specific conditions. This is due to the amplification and sequencing of only short 16S rRNA gene regions, typically providing for only family- or genus-level taxonomy. Moreover, sequencing errors often inflate the number of taxa present. Pacific Biosciences' (PacBio's) long-read technology in particular suffers from high error rates per base. Herein, we present a microbiome analysis pipeline that takes advantage of PacBio circular consensus sequencing (CCS) technology to sequence and error correct full-length bacterial 16S rRNA genes, which provides high-fidelity species-level microbiome data.
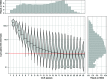
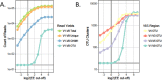
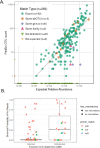
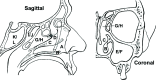
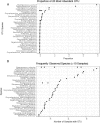
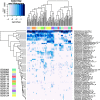
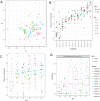
Results: Analysis of a mock community with 20 bacterial species demonstrated 100% specificity and sensitivity with regard to taxonomic classification. Examination of a 250-plus species mock community demonstrated correct species-level classification of > 90% of taxa, and relative abundances were accurately captured. The majority of the remaining taxa were demonstrated to be multiply, incorrectly, or incompletely classified. Using this methodology, we examined the microgeographic variation present among the microbiomes of six sinonasal sites, by both swab and biopsy, from the anterior nasal cavity to the sphenoid sinus from 12 subjects undergoing trans-sphenoidal hypophysectomy. We found greater variation among subjects than among sites within a subject, although significant within-individual differences were also observed. Propiniobacterium acnes (recently renamed Cutibacterium acnes) was the predominant species throughout, but was found at distinct relative abundances by site.
Conclusions: Our microbial composition analysis pipeline for single-molecule real-time 16S rRNA gene sequencing (MCSMRT, https://github.com/jpearl01/mcsmrt ) overcomes deficits of standard marker gene-based microbiome analyses by using CCS of entire 16S rRNA genes to provide increased taxonomic and phylogenetic resolution. Extensions of this approach to other marker genes could help refine taxonomic assignments of microbial species and improve reference databases, as well as strengthen the specificity of associations between microbial communities and dysbiotic states.
Keywords: 16S rRNA; Circular consensus sequencing; Database; Long-read DNA sequencing; Microbiome; Paranasal sinuses; Sinonasal.
Conflict of interest statement
Ethics approval and consent to participate
The Institutional Review Board at The University of Pennsylvania School of Medicine provided full study approval, and informed consent was obtained pre-operatively from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

