Selinexor plus low-dose bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma
- PMID: 30352784
- PMCID: PMC6302280
- DOI: 10.1182/blood-2018-06-858852
Selinexor plus low-dose bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma
Abstract
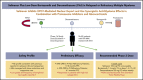
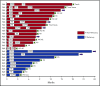
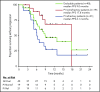
Selinexor is an oral inhibitor of the nuclear export protein exportin 1. Preclinical studies demonstrated synergistic antimyeloma activity between selinexor and proteasome inhibitors (PI) through suppression of NF-κB signaling and nuclear retention of tumor suppressor proteins. We tested selinexor in combination with low-dose bortezomib and dexamethasone (SVd) for the treatment of relapsed or refractory multiple myeloma (MM). The primary objectives of this study were to determine the safety profile, overall response rate (ORR), and a recommended phase 2 dose (RP2D) of SVd. We enrolled 42 patients to receive selinexor (60, 80, or 100 mg orally) plus bortezomib (1.3 mg/m2 subcutaneously) and dexamethasone (20 mg orally) once or twice weekly in 21- or 35-day cycles. Patients had a median of 3 (range 1-11) prior lines of therapy, and 50% were refractory to a PI. Treatment-related grade 3 or 4 adverse events reported in ≥10% of patients were thrombocytopenia (45%), neutropenia (24%), fatigue (14%), and anemia (12%). Incidence (4 patients, 10%) and grade (≤2) of peripheral neuropathy were low. The ORR for the entire population was 63%: 84% ORR for PI nonrefractory and 43% for PI-refractory patients. The median progression-free survival for all patients was 9.0 months; 17.8 months for PI nonrefractory, and 6.1 months for PI refractory. SVd treatment produced high response rates in patients with relapsed or refractory MM, including borezomib-refractory MM, with no unexpected side effects. The RP2D is selinexor (100 mg once weekly), bortezomib (1.3 mg/m2 once weekly for 4 weeks), and dexamethasone (40 mg once weekly) per 35-day cycle. This trial was registered at www.clinicaltrials.gov as #NCT02343042.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: D.W. has received consultancy fees and honoraria from Amgen, Celgene, Janssen, Sanofi, and Takeda. S.L. is Chief Scientific Advisor and a shareholder of Caelum Biosciences and sits on the Advisory Boards of Janssen and Bayer. R.K. is a stockholder in Karyopharm Therapeutics. C.P.V. has received honoraria from Johnson & Johnson, Celgene, Amgen, and Takeda. M.K. is CEO of Karyopharm Therapeutics and a stockholder in Karyopharm Therapeutics. S.S. is CSO of Karyopharm Therapeutics and a stockholder in Karyopharm Therapeutics. T.J.U. is an employee of Karyopharm Therapeutics and a stockholder in Karyopharm Therapeutics. J.J. is an employee of Karyopharm Therapeutics and a stockholder in Karyopharm Therapeutics. J.-R.S.-M. is an employee of Karyopharm Therapeutics and a stockholder in Karyopharm Therapeutics. J.S. is an employee of Karyopharm Therapeutics and a stockholder in Karyopharm Therapeutics. The remaining authors declare no competing financial interests.
Figures
References
-
- Dimopoulos MA, Richardson PG, Moreau P, Anderson KC. Current treatment landscape for relapsed and/or refractory multiple myeloma. Nat Rev Clin Oncol. 2015;12(1):42-54. - PubMed
-
- Dimopoulos MA, Moreau P, Palumbo A, et al. ; ENDEAVOR Investigators. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27-38. - PubMed
-
- Dimopoulos MA, Chen C, Spencer A, et al. . Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 2009;23(11):2147-2152. - PubMed
-
- Richardson PG, Sonneveld P, Schuster M, et al. . Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110(10):3557-3560. - PubMed
-
- Miguel JS, Weisel K, Moreau P, et al. . Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(11):1055-1066. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

