Clonal Relatedness and Mutational Differences between Upper Tract and Bladder Urothelial Carcinoma
- PMID: 30352907
- PMCID: PMC6359971
- DOI: 10.1158/1078-0432.CCR-18-2039
Clonal Relatedness and Mutational Differences between Upper Tract and Bladder Urothelial Carcinoma
Abstract
Purpose: To investigate genomic differences between urothelial carcinomas of the upper tract (UTUC) and bladder (UCB), with a focus on defining the clonal relatedness of temporally distinct tumors.
Experimental design: We prospectively sequenced tumors and matched germline DNA using targeted next-generation sequencing methods. The cohort included 195 UTUC patients and 454 UCB patients. For a subgroup of 29 patients with UTUC and a history of a subsequent UCB, both tumors were analyzed to assess their clonal relatedness.
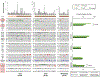
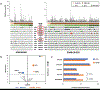
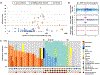
Results: With the progression to higher UTUC clinical state, there were fewer alterations in the RTK/RAS pathway but more alterations in TP53/MDM2. Compared with UCB, TP53, RB1, and ERBB2 were less frequently altered in UTUC (26% vs. 46%, 3% vs. 20%, 8% vs. 19%, respectively; Q < 0.001), whereas FGFR3 and HRAS were more frequently altered (40% vs. 26%, 12% vs. 4%, respectively; Q < 0.001). On the basis of an integrated analysis of tumor mutational burden, MSIsensor score and mutational signature, 7.2% of UTUC tumors were classified as MSI-high/MMR-deficient (MSI-H/dMMR). The risk of bladder recurrence after UTUC was significantly associated with mutations in FGFR3, KDM6A, CCND1, and TP53. Comparison of UCB with corresponding UTUC tumors from the same patient supports their clonal relatedness.
Conclusions: UTUC and UCB exhibit significant differences in the prevalence of common genomic alterations. In individual patients with a history of both tumors, UCB and UTUC were always clonally related. Genomic characterization of UTUC provides information regarding the risk of bladder recurrence and can identify tumors associated with Lynch syndrome.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures


Comment in
-
Re: Clonal Relatedness and Mutational Differences between Upper Tract and Bladder Urothelial Carcinoma.J Urol. 2019 Sep;202(3):456-457. doi: 10.1097/01.JU.0000569136.58106.66. Epub 2019 Aug 8. J Urol. 2019. PMID: 31184534 No abstract available.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. - PubMed
-
- Babjuk M, Böhle A, Burger M, Capoun O, Cohen D, Compérat EM, et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol. 2017;71:447–61. - PubMed
-
- Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol. 2000;164:1523–5. - PubMed
-
- Green DA, Rink M, Xylinas E, Matin SF, Stenzl A, Rouprêt M, et al. Urothelial carcinoma of the bladder and the upper tract: disparate twins. J Urol. 2013;189:1214–21. - PubMed
-
- Chiou HY, Chiou ST, Hsu YH, Chou YL, Tseng CH, Wei ML, et al. Incidence of transitional cell carcinoma and arsenic in drinking water: a follow-up study of 8,102 residents in an arseniasis-endemic area in northeastern Taiwan. American journal of epidemiology. 2001;153:411–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

