Dynamic coordination of two-metal-ions orchestrates λ-exonuclease catalysis
- PMID: 30353000
- PMCID: PMC6199318
- DOI: 10.1038/s41467-018-06750-9
Dynamic coordination of two-metal-ions orchestrates λ-exonuclease catalysis
Abstract
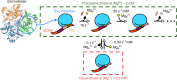
Metal ions at the active site of an enzyme act as cofactors, and their dynamic fluctuations can potentially influence enzyme activity. Here, we use λ-exonuclease as a model enzyme with two Mg2+ binding sites and probe activity at various concentrations of magnesium by single-molecule-FRET. We find that while MgA2+ and MgB2+ have similar binding constants, the dissociation rate of MgA2+ is two order of magnitude lower than that of MgB2+ due to a kinetic-barrier-difference. At physiological Mg2+ concentration, the MgB2+ ion near the 5'-terminal side of the scissile phosphate dissociates each-round of degradation, facilitating a series of DNA cleavages via fast product-release concomitant with enzyme-translocation. At a low magnesium concentration, occasional dissociation and slow re-coordination of MgA2+ result in pauses during processive degradation. Our study highlights the importance of metal-ion-coordination dynamics in correlation with the enzymatic reaction-steps, and offers insights into the origin of dynamic heterogeneity in enzymatic catalysis.
Conflict of interest statement
The authors declare no competing interests
Figures





Similar articles
-
Neutralizing mutations of carboxylates that bind metal 2 in T5 flap endonuclease result in an enzyme that still requires two metal ions.J Biol Chem. 2011 Sep 2;286(35):30878-30887. doi: 10.1074/jbc.M111.230391. Epub 2011 Jul 6. J Biol Chem. 2011. PMID: 21734257 Free PMC article.
-
Catalytic metal ions and enzymatic processing of DNA and RNA.Acc Chem Res. 2015 Feb 17;48(2):220-8. doi: 10.1021/ar500314j. Epub 2015 Jan 15. Acc Chem Res. 2015. PMID: 25590654 Review.
-
Crystal structure of λ exonuclease in complex with DNA and Ca(2+).Biochemistry. 2014 Dec 2;53(47):7415-25. doi: 10.1021/bi501155q. Epub 2014 Nov 19. Biochemistry. 2014. PMID: 25370446
-
Kinetic and magnetic resonance studies of the role of metal ions in the mechanism of Escherichia coli GDP-mannose mannosyl hydrolase, an unusual nudix enzyme.Biochemistry. 2002 Apr 9;41(14):4655-68. doi: 10.1021/bi012118d. Biochemistry. 2002. PMID: 11926828
-
Breaking the Concentration Barrier for Single-Molecule Fluorescence Measurements.Chemistry. 2018 Jan 24;24(5):1002-1009. doi: 10.1002/chem.201704065. Epub 2017 Nov 30. Chemistry. 2018. PMID: 29044746 Review.
Cited by
-
RNase H is an exo- and endoribonuclease with asymmetric directionality, depending on the binding mode to the structural variants of RNA:DNA hybrids.Nucleic Acids Res. 2022 Feb 28;50(4):1801-1814. doi: 10.1093/nar/gkab1064. Nucleic Acids Res. 2022. PMID: 34788459 Free PMC article.
-
Recruiting Mechanism and Functional Role of a Third Metal Ion in the Enzymatic Activity of 5' Structure-Specific Nucleases.J Am Chem Soc. 2020 Feb 12;142(6):2823-2834. doi: 10.1021/jacs.9b10656. Epub 2020 Jan 27. J Am Chem Soc. 2020. PMID: 31939291 Free PMC article.
-
Bioinspired Active Site with a Coordination-Adaptive Organosulfonate Ligand for Catalytic Water Oxidation at Neutral pH.J Am Chem Soc. 2023 May 31;145(21):11818-11828. doi: 10.1021/jacs.3c03415. Epub 2023 May 17. J Am Chem Soc. 2023. PMID: 37196315 Free PMC article.
-
A Proofreading Mutation with an Allosteric Effect Allows a Cluster of SARS-CoV-2 Viruses to Rapidly Evolve.Mol Biol Evol. 2023 Oct 4;40(10):msad209. doi: 10.1093/molbev/msad209. Mol Biol Evol. 2023. PMID: 37738143 Free PMC article.
-
A metal ion-dependent conformational switch modulates activity of the Plasmodium M17 aminopeptidase.J Biol Chem. 2022 Jul;298(7):102119. doi: 10.1016/j.jbc.2022.102119. Epub 2022 Jun 9. J Biol Chem. 2022. PMID: 35691342 Free PMC article.
References
-
- Cowan J. A. The Biological Chemistry of Magnesium (VCH, New York, 1995).
-
- Sigel H. Metal Ions in Biological Systems (Marcel Dekker, New York, 1974).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

