A microfabricated nerve-on-a-chip platform for rapid assessment of neural conduction in explanted peripheral nerve fibers
- PMID: 30353009
- PMCID: PMC6199302
- DOI: 10.1038/s41467-018-06895-7
A microfabricated nerve-on-a-chip platform for rapid assessment of neural conduction in explanted peripheral nerve fibers
Abstract
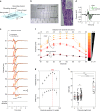
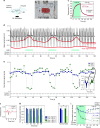
Peripheral nerves are anisotropic and heterogeneous neural tissues. Their complex physiology restricts realistic in vitro models, and high resolution and selective probing of axonal activity. Here, we present a nerve-on-a-chip platform that enables rapid extracellular recording and axonal tracking of action potentials collected from tens of myelinated fibers. The platform consists of microfabricated stimulation and recording microchannel electrode arrays. First, we identify conduction velocities of action potentials traveling through the microchannel and propose a robust data-sorting algorithm using velocity selective recording. We optimize channel geometry and electrode spacing to enhance the algorithm reliability. Second, we demonstrate selective heat-induced neuro-inhibition of peripheral nerve activity upon local illumination of a conjugated polymer (P3HT) blended with a fullerene derivative (PCBM) coated on the floor of the microchannel. We demonstrate the nerve-on-a-chip platform is a versatile tool to optimize the design of implantable peripheral nerve interfaces and test selective neuromodulation techniques ex vivo.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Experimental validation of the nerve conduction velocity selective recording technique using a multi-contact cuff electrode.Med Eng Phys. 2009 Dec;31(10):1261-70. doi: 10.1016/j.medengphy.2009.08.005. Epub 2009 Sep 16. Med Eng Phys. 2009. PMID: 19762269
-
In vivo electrical conductivity across critical nerve gaps using poly(3,4-ethylenedioxythiophene)-coated neural interfaces.Plast Reconstr Surg. 2010 Dec;126(6):1865-1873. doi: 10.1097/PRS.0b013e3181f61848. Plast Reconstr Surg. 2010. PMID: 20700080
-
The electrophysiological consequences of electrode impalement of peripheral nerves in the rat.Brain Res. 1993 Dec 24;631(2):221-6. doi: 10.1016/0006-8993(93)91538-4. Brain Res. 1993. PMID: 8131050
-
[Test for analysing nerve conduction velocity].Rinsho Shinkeigaku. 1991 Dec;31(12):1326-9. Rinsho Shinkeigaku. 1991. PMID: 1817800 Review. Japanese.
-
The Molecular and Morphologic Structures That Make Saltatory Conduction Possible in Peripheral Nerve.J Neuropathol Exp Neurol. 2017 Apr 1;76(4):255-257. doi: 10.1093/jnen/nlx013. J Neuropathol Exp Neurol. 2017. PMID: 28340093 Review.
Cited by
-
Biomimetic Strategies for Peripheral Nerve Injury Repair: An Exploration of Microarchitecture and Cellularization.Biomed Mater Devices. 2023 Mar;1(1):21-37. doi: 10.1007/s44174-022-00039-8. Epub 2022 Oct 3. Biomed Mater Devices. 2023. PMID: 38343513 Free PMC article.
-
Neural modulation with photothermally active nanomaterials.Nat Rev Bioeng. 2023 Mar;1(3):193-207. doi: 10.1038/s44222-023-00022-y. Epub 2023 Jan 31. Nat Rev Bioeng. 2023. PMID: 39221032 Free PMC article.
-
Nerve-on-a-Chip Derived Biomimicking Microfibers for Peripheral Nerve Regeneration.Adv Sci (Weinh). 2023 Jul;10(20):e2207536. doi: 10.1002/advs.202207536. Epub 2023 Apr 29. Adv Sci (Weinh). 2023. PMID: 37119478 Free PMC article.
-
A human-on-a-chip approach to tackling rare diseases.Drug Discov Today. 2019 Nov;24(11):2139-2151. doi: 10.1016/j.drudis.2019.08.001. Epub 2019 Aug 11. Drug Discov Today. 2019. PMID: 31412288 Free PMC article. Review.
-
A flexible, thin-film microchannel electrode array device for selective subdiaphragmatic vagus nerve recording.Microsyst Nanoeng. 2024 Jan 23;10:16. doi: 10.1038/s41378-023-00637-6. eCollection 2024. Microsyst Nanoeng. 2024. PMID: 38264708 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

