Locus Coeruleus as a vigilance centre for active inspiration and expiration in rats
- PMID: 30353035
- PMCID: PMC6199338
- DOI: 10.1038/s41598-018-34047-w
Locus Coeruleus as a vigilance centre for active inspiration and expiration in rats
Abstract
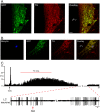
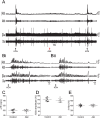
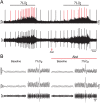
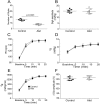
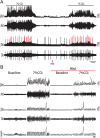
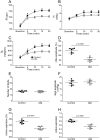
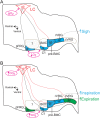
At rest, inspiration is an active process while expiration is passive. However, high chemical drive (hypercapnia or hypoxia) activates central and peripheral chemoreceptors triggering reflex increases in inspiration and active expiration. The Locus Coeruleus contains noradrenergic neurons (A6 neurons) that increase their firing frequency when exposed to hypercapnia and hypoxia. Using recently developed neuronal hyperpolarising technology in conscious rats, we tested the hypothesis that A6 neurons are a part of a vigilance centre for controlling breathing under high chemical drive and that this includes recruitment of active inspiration and expiration in readiness for flight or fight. Pharmacogenetic inhibition of A6 neurons was without effect on resting and on peripheral chemoreceptors-evoked inspiratory, expiratory and ventilatory responses. On the other hand, the number of sighs evoked by systemic hypoxia was reduced. In the absence of peripheral chemoreceptors, inhibition of A6 neurons during hypercapnia did not affect sighing, but reduced both the magnitude and incidence of active expiration, and the frequency and amplitude of inspiration. These changes reduced pulmonary ventilation. Our data indicated that A6 neurons exert a CO2-dependent modulation of expiratory drive. The data also demonstrate that A6 neurons contribute to the CO2-evoked increases in the inspiratory motor output and hypoxia-evoked sighing.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Lindvall O, Bjorklund A. The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand Suppl. 1974;412:1–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

