The Sema3A receptor Plexin-A1 suppresses supernumerary axons through Rap1 GTPases
- PMID: 30353093
- PMCID: PMC6199275
- DOI: 10.1038/s41598-018-34092-5
The Sema3A receptor Plexin-A1 suppresses supernumerary axons through Rap1 GTPases
Abstract
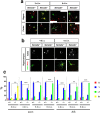
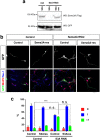
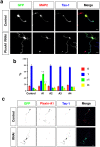
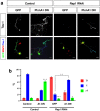
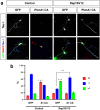
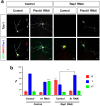
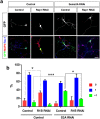
The highly conserved Rap1 GTPases perform essential functions during neuronal development. They are required for the polarity of neuronal progenitors and neurons as well as for neuronal migration in the embryonic brain. Neuronal polarization and axon formation depend on the precise temporal and spatial regulation of Rap1 activity by guanine nucleotide exchange factors (GEFs) and GTPases-activating proteins (GAPs). Several Rap1 GEFs have been identified that direct the formation of axons during cortical and hippocampal development in vivo and in cultured neurons. However little is known about the GAPs that limit the activity of Rap1 GTPases during neuronal development. Here we investigate the function of Sema3A and Plexin-A1 as a regulator of Rap1 GTPases during the polarization of hippocampal neurons. Sema3A was shown to suppress axon formation when neurons are cultured on a patterned substrate. Plexin-A1 functions as the signal-transducing subunit of receptors for Sema3A and displays GAP activity for Rap1 GTPases. We show that Sema3A and Plexin-A1 suppress the formation of supernumerary axons in cultured neurons, which depends on Rap1 GTPases.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Shah B, et al. Rap1 GTPases Are Master Regulators of Neural Cell Polarity in the Developing Neocortex. Cereb Cortex. 2017;27:1253–1269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

