Inhibiting ex-vivo Th17 responses in Ankylosing Spondylitis by targeting Janus kinases
- PMID: 30353145
- PMCID: PMC6199284
- DOI: 10.1038/s41598-018-34026-1
Inhibiting ex-vivo Th17 responses in Ankylosing Spondylitis by targeting Janus kinases
Abstract
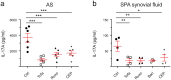
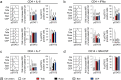
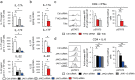
Treatment options for Ankylosing Spondylitis (AS) are still limited. The T helper cell 17 (Th17) pathway has emerged as a major driver of disease pathogenesis and a good treatment target. Janus kinases (JAK) are key transducers of cytokine signals in Th17 cells and therefore promising targets for the treatment of AS. Here we investigate the therapeutic potential of four different JAK inhibitors on cells derived from AS patients and healthy controls, cultured in-vitro under Th17-promoting conditions. Levels of IL-17A, IL-17F, IL-22, GM-CSF and IFNγ were assessed by ELISA and inhibitory effects were investigated with Phosphoflow. JAK1/2/3 and TYK2 were silenced in CD4+ T cells with siRNA and effects analyzed by ELISA (IL-17A, IL-17F and IL-22), Western Blot, qPCR and Phosphoflow. In-vitro inhibition of CD4+ T lymphocyte production of multiple Th17 cytokines (IL-17A, IL-17F and IL-22) was achieved with JAK inhibitors of differing specificity, as well as by silencing of JAK1-3 and Tyk2, without impacting on cell viability or proliferation. Our preclinical data suggest JAK inhibitors as promising candidates for therapeutic trials in AS, since they can inhibit multiple Th17 cytokines simultaneously. Improved targeting of TYK2 or other JAK isoforms may confer tailored effects on Th17 responses in AS.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

