Ionic and cellular mechanisms underlying TBX5/PITX2 insufficiency-induced atrial fibrillation: Insights from mathematical models of human atrial cells
- PMID: 30353147
- PMCID: PMC6199257
- DOI: 10.1038/s41598-018-33958-y
Ionic and cellular mechanisms underlying TBX5/PITX2 insufficiency-induced atrial fibrillation: Insights from mathematical models of human atrial cells
Abstract
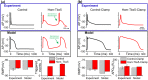
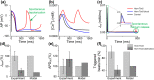
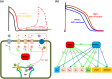
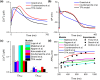
Transcription factors TBX5 and PITX2 involve in the regulation of gene expression of ion channels and are closely associated with atrial fibrillation (AF), the most common cardiac arrhythmia in developed countries. The exact cellular and molecular mechanisms underlying the increased susceptibility to AF in patients with TBX5/PITX2 insufficiency remain unclear. In this study, we have developed and validated a novel human left atrial cellular model (TPA) based on the ten Tusscher-Panfilov ventricular cell model to systematically investigate how electrical remodeling induced by TBX5/PITX2 insufficiency leads to AF. Using our TPA model, we have demonstrated that spontaneous diastolic depolarization observed in atrial myocytes with TBX5-deletion can be explained by altered intracellular calcium handling and suppression of inward-rectifier potassium current (IK1). Additionally, our computer simulation results shed new light on the novel cellular mechanism underlying AF by indicating that the imbalance between suppressed outward current IK1 and increased inward sodium-calcium exchanger current (INCX) resulted from SR calcium leak leads to spontaneous depolarizations. Furthermore, our simulation results suggest that these arrhythmogenic triggers can be potentially suppressed by inhibiting sarcoplasmic reticulum (SR) calcium leak and reversing remodeled IK1. More importantly, this study has clinically significant implications on the drugs used for maintaining SR calcium homeostasis, whereby drugs such as dantrolene may confer significant improvement for the treatment of AF patients with TBX5/PITX2 insufficiency.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
In silico investigation of the mechanisms underlying atrial fibrillation due to impaired Pitx2.PLoS Comput Biol. 2020 Feb 25;16(2):e1007678. doi: 10.1371/journal.pcbi.1007678. eCollection 2020 Feb. PLoS Comput Biol. 2020. PMID: 32097431 Free PMC article.
-
A calcium transport mechanism for atrial fibrillation in Tbx5-mutant mice.Elife. 2019 Mar 21;8:e41814. doi: 10.7554/eLife.41814. Elife. 2019. PMID: 30896405 Free PMC article.
-
An atrial fibrillation-associated regulatory region modulates cardiac Tbx5 levels and arrhythmia susceptibility.Elife. 2023 Jan 30;12:e80317. doi: 10.7554/eLife.80317. Elife. 2023. PMID: 36715501 Free PMC article.
-
Calcium-mediated cellular triggered activity in atrial fibrillation.J Physiol. 2017 Jun 15;595(12):4001-4008. doi: 10.1113/JP273048. Epub 2017 Mar 22. J Physiol. 2017. PMID: 28181690 Free PMC article. Review.
-
Understanding PITX2-Dependent Atrial Fibrillation Mechanisms through Computational Models.Int J Mol Sci. 2021 Jul 19;22(14):7681. doi: 10.3390/ijms22147681. Int J Mol Sci. 2021. PMID: 34299303 Free PMC article. Review.
Cited by
-
The Antimalarial Chloroquine Reduces the Burden of Persistent Atrial Fibrillation.Front Pharmacol. 2019 Nov 27;10:1392. doi: 10.3389/fphar.2019.01392. eCollection 2019. Front Pharmacol. 2019. PMID: 31827438 Free PMC article.
-
Computational modeling of cardiac electrophysiology and arrhythmogenesis: toward clinical translation.Physiol Rev. 2024 Jul 1;104(3):1265-1333. doi: 10.1152/physrev.00017.2023. Epub 2023 Dec 28. Physiol Rev. 2024. PMID: 38153307 Free PMC article. Review.
-
Transcriptional factors in calcium mishandling and atrial fibrillation development.Pflugers Arch. 2021 Aug;473(8):1177-1197. doi: 10.1007/s00424-021-02553-y. Epub 2021 May 18. Pflugers Arch. 2021. PMID: 34003377 Free PMC article. Review.
-
Modeling and simulation of cardiac electric activity in a human cardiac tissue with multiple ischemic zones.J Math Biol. 2019 Sep;79(4):1551-1586. doi: 10.1007/s00285-019-01403-x. Epub 2019 Jul 27. J Math Biol. 2019. PMID: 31352562
-
PITX2 upregulation increases the risk of chronic atrial fibrillation in a dose-dependent manner by modulating IKs and ICaL -insights from human atrial modelling.Ann Transl Med. 2020 Mar;8(5):191. doi: 10.21037/atm.2020.01.90. Ann Transl Med. 2020. PMID: 32309338 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

