Preliminary report of a single-channel applicator in high dose rate afterloading brachytherapy for cervical cancer
- PMID: 30353607
- PMCID: PMC6272109
- DOI: 10.1111/cas.13845
Preliminary report of a single-channel applicator in high dose rate afterloading brachytherapy for cervical cancer
Abstract
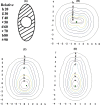
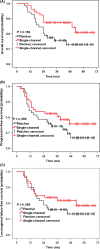
The aim of this study was to evaluate whether a patented single-channel applicator, which was modified from the traditional tandem applicator and wrapped with an oval-shield alloy around the source channel, has the same clinical efficacy and safety as the standard Fletcher-type applicator in high dose rate (HDR) brachytherapy for carcinoma of the cervix. Between December 2011 and February 2017, 299 patients with pathologically confirmed International Federation of Gynecology and Obstetrics (2009) stage Ib2-IVa cervical cancer were recruited to the trial and finished the allocated intervention. Of the first 151 patients, 71 were allocated to the Fletcher group and 80 to the single-channel group, satisfying the criteria for a preliminary analysis. All but 3 patients were treated with concurrent cisplatin chemotherapy and external beam radiotherapy followed by HDR brachytherapy. The 2-year overall survival, progression-free survival, and locoregional failure-free survival was 80.3%, 77.5%, and 78.9%, respectively, for the Fletcher group, and 86.3%, 82.5%, and 83.8%, respectively, for the single-channel group. The seriousness of acute treatment-related toxicities was similar in the 2 groups. The cumulative rate of late rectal complications of grade 3-4 in the Fletcher group and the single-channel group was 2.8% and 2.5%, respectively. The cumulative rate of grade 3 bladder complications was 2.8% for the Fletcher group and 1.3% for the single-channel group. The preliminary results of our study show that the patented single-channel intracavitary applicator might be able to provide protection for the rectum and bladder and seems to have the same clinical efficacy as the standard Fletcher-type 3-channel applicator in HDR brachytherapy for carcinoma of the cervix. This trial was registered with the Chinese Clinical Trial Registry (registration no. ChiCTR-TRC-12002321).
Keywords: cervical carcinoma; clinical trial; concurrent chemoradiotherapy; high dose rate brachytherapy; radiotherapy.
© 2018 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures

References
-
- Sankaranarayanan R. Overview of cervical cancer in the developing world. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S205‐S210. - PubMed
-
- Gaffney DK, Erickson‐Wittmann BA, Jhingran A, et al. ACR Appropriateness Criteria(R) on advanced cervical cancer expert panel on radiation oncology‐gynecology. Int J Radiat Oncol Biol Phys. 2011;81(3):609‐614. - PubMed
-
- Al Feghali KA, Elshaikh MA. Why brachytherapy boost is the treatment of choice for most women with locally advanced cervical carcinoma? Brachytherapy. 2016;15(2):191‐199. - PubMed

