Reduction of cysteine-S-protecting groups by triisopropylsilane
- PMID: 30353614
- PMCID: PMC6773273
- DOI: 10.1002/psc.3130
Reduction of cysteine-S-protecting groups by triisopropylsilane
Abstract
Triisopropylsilane (TIS), a hindered hydrosilane, has long been utilized as a cation scavenger for the removal of amino acid protecting groups during peptide synthesis. However, its ability to actively remove S-protecting groups by serving as a reductant has largely been mischaracterized by the peptide community. Here, we provide strong evidence that TIS can act as a reducing agent to facilitate the removal of acetamidomethyl (Acm), 4-methoxybenzyl (Mob), and tert-butyl (But ) protecting groups from cysteine (Cys) residues in the presence of trifluoroacetic acid (TFA) at 37 °C. The lability of the Cys protecting groups in TFA/TIS (98/2) in this study are in the order: Cys(Mob) > Cys(Acm) > Cys(But ), with Cys(Mob) being especially labile. Unexpectedly, we found that TIS promoted disulfide formation in addition to aiding in the removal of the protecting group. Our results raise the possibility of using TIS in orthogonal deprotection strategies of Cys-protecting groups following peptide synthesis as TIS can be viewed as a potential deprotection agent instead of merely a scavenger in deprotection cocktails based on our results. We also tested other common scavengers under these reaction conditions and found that thioanisole and triethylsilane were similarly effective as TIS in enhancing deprotection and catalyzing disulfide formation. Our findings reported herein show that careful consideration should be given to the type of scavenger used when it is desirable to preserve the Cys-protecting group. Additional consideration should be given to the concentration of scavenger, temperature of the reaction, and reaction time.
© 2018 European Peptide Society and John Wiley & Sons, Ltd.
Figures

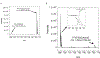
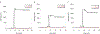



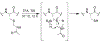
Similar articles
-
Facile removal of 4-methoxybenzyl protecting group from selenocysteine.J Pept Sci. 2019 Oct;25(10):e3209. doi: 10.1002/psc.3209. Epub 2019 Aug 13. J Pept Sci. 2019. PMID: 31410953 Free PMC article.
-
2,2'-Dipyridyl diselenide: A chemoselective tool for cysteine deprotection and disulfide bond formation.J Pept Sci. 2020 Mar;26(3):e3236. doi: 10.1002/psc.3236. Epub 2019 Dec 19. J Pept Sci. 2020. PMID: 31856422 Free PMC article.
-
Removal of the 5-nitro-2-pyridine-sulfenyl protecting group from selenocysteine and cysteine by ascorbolysis.J Pept Sci. 2016 Sep;22(9):571-6. doi: 10.1002/psc.2908. Epub 2016 Aug 2. J Pept Sci. 2016. PMID: 27480992 Free PMC article.
-
[A novel synthetic approach of tryptophan-containing cystine peptides by regioselective disulfide bond-forming reaction using the silyl chloride-sulfoxide system].Yakugaku Zasshi. 2000 Feb;120(2):197-205. doi: 10.1248/yakushi1947.120.2_197. Yakugaku Zasshi. 2000. PMID: 10689966 Review. Japanese.
-
New disulfide bond-forming reactions for peptide and protein synthesis.Braz J Med Biol Res. 1994 Dec;27(12):2733-44. doi: 10.1002/chin.199620250. Braz J Med Biol Res. 1994. PMID: 7549997 Review.
Cited by
-
Tetrahydroimidazo[1,2-a]pyrazine Derivatives: Synthesis and Evaluation as Gαq -Protein Ligands.Chemistry. 2020 Oct 1;26(55):12615-12623. doi: 10.1002/chem.202001446. Epub 2020 Sep 7. Chemistry. 2020. PMID: 32428383 Free PMC article.
-
Facile removal of 4-methoxybenzyl protecting group from selenocysteine.J Pept Sci. 2019 Oct;25(10):e3209. doi: 10.1002/psc.3209. Epub 2019 Aug 13. J Pept Sci. 2019. PMID: 31410953 Free PMC article.
-
S-Protected Cysteine Sulfoxide-Enabled Tryptophan-Selective Modification with Application to Peptide Lipidation.ACS Med Chem Lett. 2022 Jun 14;13(7):1125-1130. doi: 10.1021/acsmedchemlett.2c00161. eCollection 2022 Jul 14. ACS Med Chem Lett. 2022. PMID: 35859873 Free PMC article.
-
Incorporation of a Highly Reactive Oxalyl Thioester-Based Interacting Handle into Proteins.Org Lett. 2023 Jul 14;25(27):5117-5122. doi: 10.1021/acs.orglett.3c01846. Epub 2023 Jun 29. Org Lett. 2023. PMID: 37384828 Free PMC article.
-
Challenges and Perspectives in Chemical Synthesis of Highly Hydrophobic Peptides.Front Bioeng Biotechnol. 2020 Mar 4;8:162. doi: 10.3389/fbioe.2020.00162. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32195241 Free PMC article. Review.
References
-
- Pearson DA, Blanchette M, Baker ML, Guindon CA. Trialkylsilanes as scavengers for the trifluoroacetic acid deblocking of protecting groups in peptide synthesis. Tetrahedron Lett 1989; 30: 2739–2742.
-
- Isidro-Llobet A, Alvarez M, Albericio F Amino acid-protecting groups. Chem Rev 2009; 109: 2455–2504. - PubMed
-
- Moroder L, Musiol HJ, Schaschke N, Chen L, Hargittai B, Barany G. In Houben-Weyl, Synthesis of Peptides and Peptidomimetics; Goodman M, Felix A, Moroder L, Toniolo C. Eds.; Georg Thieme Verlag: Stuttgart, 2004; pp 384–423.
-
- Larson GL, Halides M. Silicon-based reducing agents. Supplement to the Gelest Catalog available on-line at www gelest com. 2008.
-
- Pesti J, Larson GL. Tetramethyldisiloxane: A practical organosilane reducing agent. Org. Process Res. Dev 2016; 20: 1164–1181.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
