Ovarian Carcinoma-Associated Mesenchymal Stem Cells Arise from Tissue-Specific Normal Stroma
- PMID: 30353617
- PMCID: PMC6392140
- DOI: 10.1002/stem.2932
Ovarian Carcinoma-Associated Mesenchymal Stem Cells Arise from Tissue-Specific Normal Stroma
Abstract
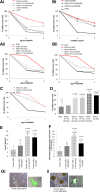
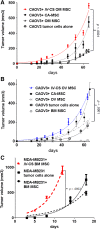
Carcinoma-associated mesenchymal stem cells (CA-MSCs) are critical stromal progenitor cells within the tumor microenvironment (TME). We previously demonstrated that CA-MSCs differentially express bone morphogenetic protein family members, promote tumor cell growth, increase cancer "stemness," and chemotherapy resistance. Here, we use RNA sequencing of normal omental MSCs and ovarian CA-MSCs to demonstrate global changes in CA-MSC gene expression. Using these expression profiles, we create a unique predictive algorithm to classify CA-MSCs. Our classifier accurately distinguishes normal omental, ovary, and bone marrow MSCs from ovarian cancer CA-MSCs. Suggesting broad applicability, the model correctly classifies pancreatic and endometrial cancer CA-MSCs and distinguishes cancer associated fibroblasts from CA-MSCs. Using this classifier, we definitively demonstrate ovarian CA-MSCs arise from tumor mediated reprograming of local tissue MSCs. Although cancer cells alone cannot induce a CA-MSC phenotype, the in vivo ovarian TME can reprogram omental or ovary MSCs to protumorigenic CA-MSCs (classifier score of >0.96). In vitro studies suggest that both tumor secreted factors and hypoxia are critical to induce the CA-MSC phenotype. Interestingly, although the breast cancer TME can reprogram bone marrow MSCs into CA-MSCs, the ovarian TME cannot, demonstrating for the first time that tumor mediated CA-MSC conversion is tissue and cancer type dependent. Together these findings (a) provide a critical tool to define CA-MSCs and (b) highlight cancer cell influence on distinct normal tissues providing powerful insights into the mechanisms underlying cancer specific metastatic niche formation. Stem Cells 2019;37:257-269.
Keywords: Hypoxia; Mesenchymal stem cell; Ovarian cancer; Tumor microenvironment.
© 2018 The Authors. Stem Cells published by Wiley Periodicals, Inc. on behalf of AlphaMed Press 2018.
Conflict of interest statement
D.B. declared research support from Roche‐Genentech and AstraZeneca. R.B. declared a leadership position with Tradewind Biosciences and two patents with the University of Michigan. The other authors indicated no potential conflicts of interest.
Figures





Similar articles
-
Carcinoma-Associated Mesenchymal Stem/Stromal Cells: Architects of the Pro-tumorigenic Tumor Microenvironment.Stem Cells. 2022 Aug 25;40(8):705-715. doi: 10.1093/stmcls/sxac036. Stem Cells. 2022. PMID: 35583414 Free PMC article. Review.
-
Human carcinoma-associated mesenchymal stem cells promote ovarian cancer chemotherapy resistance via a BMP4/HH signaling loop.Oncotarget. 2016 Feb 9;7(6):6916-32. doi: 10.18632/oncotarget.6870. Oncotarget. 2016. PMID: 26755648 Free PMC article.
-
Human ovarian carcinoma–associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production.J Clin Invest. 2011 Aug;121(8):3206-19. doi: 10.1172/JCI45273. J Clin Invest. 2011. PMID: 21737876 Free PMC article.
-
Mesenchymal Stem Cells in the Tumor Microenvironment.Adv Exp Med Biol. 2020;1234:31-42. doi: 10.1007/978-3-030-37184-5_3. Adv Exp Med Biol. 2020. PMID: 32040853 Review.
-
Microenvironment in neuroblastoma: isolation and characterization of tumor-derived mesenchymal stromal cells.BMC Cancer. 2018 Nov 27;18(1):1176. doi: 10.1186/s12885-018-5082-2. BMC Cancer. 2018. PMID: 30482160 Free PMC article.
Cited by
-
Carcinoma-Associated Mesenchymal Stem/Stromal Cells: Architects of the Pro-tumorigenic Tumor Microenvironment.Stem Cells. 2022 Aug 25;40(8):705-715. doi: 10.1093/stmcls/sxac036. Stem Cells. 2022. PMID: 35583414 Free PMC article. Review.
-
The Capacity of High-Grade Serous Ovarian Cancer Cells to Form Multicellular Structures Spontaneously along Disease Progression Correlates with Their Orthotopic Tumorigenicity in Immunosuppressed Mice.Cancers (Basel). 2020 Mar 16;12(3):699. doi: 10.3390/cancers12030699. Cancers (Basel). 2020. PMID: 32188032 Free PMC article.
-
Exosomes Produced by Mesenchymal Stem Cells Drive Differentiation of Myeloid Cells into Immunosuppressive M2-Polarized Macrophages in Breast Cancer.J Immunol. 2019 Dec 15;203(12):3447-3460. doi: 10.4049/jimmunol.1900692. Epub 2019 Nov 8. J Immunol. 2019. PMID: 31704881 Free PMC article.
-
Role of cancer-associated mesenchymal stem cells in the tumor microenvironment: A review.Tzu Chi Med J. 2022 Aug 1;35(1):24-30. doi: 10.4103/tcmj.tcmj_138_22. eCollection 2023 Jan-Mar. Tzu Chi Med J. 2022. PMID: 36866340 Free PMC article. Review.
-
Modeling the Tumor Microenvironment of Ovarian Cancer: The Application of Self-Assembling Biomaterials.Cancers (Basel). 2021 Nov 16;13(22):5745. doi: 10.3390/cancers13225745. Cancers (Basel). 2021. PMID: 34830897 Free PMC article. Review.
References
-
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973–2010), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013, based on the November 2012; submission.
-
- Musrap N, Diamandis EP. Revisiting the complexity of the ovarian cancer microenvironment—Clinical implications for treatment strategies. Mol Cancer Res 2012;10:1254–1264. - PubMed
-
- da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post‐natal organs and tissues. J Cell Sci 2006;119:2204–2213. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

