The glymphatic pathway in neurological disorders
- PMID: 30353860
- PMCID: PMC6261373
- DOI: 10.1016/S1474-4422(18)30318-1
The glymphatic pathway in neurological disorders
Abstract
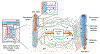
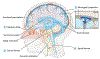
Background: The glymphatic (glial-lymphatic) pathway is a fluid-clearance pathway identified in the rodent brain in 2012. This pathway subserves the flow of CSF into the brain along arterial perivascular spaces and subsequently into the brain interstitium, facilitated by aquaporin 4 (AQP4) water channels. The pathway then directs flow towards the venous perivascular and perineuronal spaces, ultimately clearing solutes from the neuropil into meningeal and cervical lymphatic drainage vessels. In rodents, the glymphatic pathway is predominantly active during sleep, when the clearance of harmful metabolites such as amyloid β (Aβ) increases two-fold relative to the waking state. Glymphatic dysfunction, probably related to perturbed AQP4 expression, has been shown in animal models of traumatic brain injury, Alzheimer's disease, and stroke. The recent characterisations of the glymphatic and meningeal lymphatic systems in rodents and in humans call for revaluation of the anatomical routes for CSF-interstitial fluid flow and the physiological role that these pathways play in CNS health.
Recent developments: Several features of the glymphatic and meningeal lymphatic systems have been shown to be present in humans. MRI scans with intrathecally administered contrast agent show that CSF flows along pathways that closely resemble the glymphatic system outlined in rodents. Furthermore, PET studies have revealed that Aβ accumulates in the healthy brain after a single night of sleep deprivation, suggesting that the human glymphatic pathway might also be primarily active during sleep. Other PET studies have shown that CSF clearance of Aβ and tau tracers is reduced in patients with Alzheimer's disease compared with healthy controls. The observed reduction in CSF clearance was associated with increasing grey-matter concentrations of Aβ in the human brain, consistent with findings in mice showing that decreased glymphatic function leads to Aβ accumulation. Altered AQP4 expression is also evident in brain tissue from patients with Alzheimer's disease or normal pressure hydrocephalus; glymphatic MRI scans of patients with normal pressure hydrocephalus show reduced CSF tracer entry and clearance. WHERE NEXT?: Research is needed to confirm whether specific factors driving glymphatic flow in rodents also apply to humans. Longitudinal imaging studies evaluating human CSF dynamics will determine whether a causal link exists between reduced brain solute clearance and the development of neurodegenerative diseases. Assessment of glymphatic function after stroke or traumatic brain injury could identify whether this function correlates with neurological recovery. New insights into how behaviour and genetics modify glymphatic function, and how this function decompensates in disease, should lead to the development of new preventive and diagnostic tools and novel therapeutic targets.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare no conflict of interest.
Figures


Comment in
-
Making Maiken Nedergaard.Lancet Neurol. 2018 Nov;17(11):935. doi: 10.1016/S1474-4422(18)30365-X. Lancet Neurol. 2018. PMID: 30353865 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

