The Importance of Imaging in Radiation Oncology for National Clinical Trials Network Protocols
- PMID: 30353882
- PMCID: PMC6510266
- DOI: 10.1016/j.ijrobp.2018.08.039
The Importance of Imaging in Radiation Oncology for National Clinical Trials Network Protocols
Abstract
Imaging is essential in successfully executing radiation therapy (RT) in oncology clinical trials. As technically sophisticated diagnostic imaging and RT were incorporated into trials, quality assurance in the National Clinical Trials Network groups entered a new era promoting image acquisition and review. Most trials involving RT require pre- and post-therapy imaging for target validation and outcome assessment. The increasing real-time (before and during therapy) imaging and RT object reviews are to ensure compliance with trial objectives. Objects easily transmit digitally for review from anywhere in the world. Physician interpretation of imaging and image application to RT treatment plans is essential for optimal trial execution. Imaging and RT data sets are used to credential RT sites to confirm investigator and institutional ability to meet trial target volume delineation and delivery requirements. Real-time imaging and RT object reviews can be performed multiple times during a trial to assess response to therapy and application of RT objects. This process has matured into an effective data management mechanism. When necessary, site and study investigators review objects together through web media technologies to ensure the patient is enrolled on the appropriate trial and the intended RT is planned and executed in a trial-compliant manner. Real-time imaging review makes sure: (1) the patient is entered and eligible for the trial, (2) the patient meets trial-specific adaptive therapy requirements, if applicable, and (3) the intended RT is according to trial guidelines. This review ensures the study population is uniform and the results are believable and can be applied to clinical practice.
Copyright © 2018. Published by Elsevier Inc.
Conflict of interest statement
Conflict of Interest Statements
Ms. Bishop-Jodoin has nothing to disclose.
Dr. Laurie has nothing to disclose.
Ms. O’Meara has nothing to disclose.
Ms. Davis has nothing to disclose.
Dr. Bogart has nothing to disclose.
Dr. Kalapurakal has nothing to disclose.
Dr. Siegel has nothing to disclose.
Dr. Chakravarthy has nothing to disclose.
Dr. Okunieff has nothing to disclose.
Dr. Haffty has nothing to disclose.
Dr. Michalski has nothing to disclose.
Dr. Ulin has nothing to disclose.
Dr. Zhang has nothing to disclose.
Dr. Rosen has nothing to disclose.
Dr. Schwartz has nothing to disclose.
Dr. Moni has nothing to disclose.
Dr. Cicchetti has nothing to disclose.
Figures

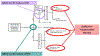
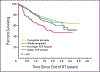
References
-
- Weiner M, Leventhal B, Brecher M, et al. Randomized study of intensive MOPP-ABVD with or without low-dose total-nodal radiation therapy in the treatment of stages IIB, IIIA2, IIIB and IV Hodgkin’s disease in pediatric patients: a Pediatric Oncology Group study. J Clin Oncol. 1997; 15(8): 2769–2779. doi: 10.1200/JCO.1997.15.8.2769. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

