Early enteral nutrition within 24 hours of lower gastrointestinal surgery versus later commencement for length of hospital stay and postoperative complications
- PMID: 30353940
- PMCID: PMC6517065
- DOI: 10.1002/14651858.CD004080.pub3
Early enteral nutrition within 24 hours of lower gastrointestinal surgery versus later commencement for length of hospital stay and postoperative complications
Update in
-
Early enteral nutrition within 24 hours of lower gastrointestinal surgery versus later commencement for length of hospital stay and postoperative complications.Cochrane Database Syst Rev. 2019 Jul 22;7(7):CD004080. doi: 10.1002/14651858.CD004080.pub4. Cochrane Database Syst Rev. 2019. PMID: 31329285 Free PMC article.
Abstract
Background: This is an update of the review last published in 2011. It focuses on early postoperative enteral nutrition after lower gastrointestinal surgery. Traditional management consisted of 'nil by mouth', where patients receive fluids followed by solids after bowel function has returned. Although several trials have reported lower incidence of infectious complications and faster wound healing upon early feeding, other trials have shown no effect. The immediate advantage of energy intake (carbohydrates, protein or fat) could enhance recovery with fewer complications, and this warrants a systematic evaluation.
Objectives: To evaluate whether early commencement of postoperative enteral nutrition (within 24 hours), oral intake and any kind of tube feeding (gastric, duodenal or jejunal), compared with traditional management (delayed nutritional supply) is associated with a shorter length of hospital stay (LoS), fewer complications, mortality and adverse events in patients undergoing lower gastrointestinal surgery (distal to the ligament of Treitz).
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library 2017, issue 10), Ovid MEDLINE (1950 to 15 November 2017), Ovid Embase (1974 to 15 November 2017). We also searched for ongoing trials in ClinicalTrials.gov and World Health Organization International Clinical Trials Registry Platform (15 November 2017). We handsearched reference lists of identified studies and previous systematic reviews.
Selection criteria: We included randomised controlled trials (RCT) comparing early commencement of enteral nutrition (within 24 hours) with no feeding in adult participants undergoing lower gastrointestinal surgery.
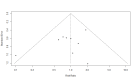
Data collection and analysis: Two review authors independently assessed study quality using the Cochrane 'Risk of bias' tool tailored to this review and extracted data. Data analyses were conducted according to the Cochrane recommendations.We rated the quality of evidence according to GRADE.Primary outcomes were LoS and postoperative complications (wound infections, intraabdominal abscesses, anastomotic dehiscence, pneumonia).Secondary outcomes were: mortality, adverse events (nausea, vomiting), and quality of life (QoL).LoS was estimated using mean difference (MD (presented as mean +/- SD). For other outcomes we estimated the common risk ratio (RR) and calculated the associated 95% confidence intervals. For analysis, we used an inverse-variance random-effects model for the primary outcome (LoS) and Mantel-Haenszel random-effects models for the secondary outcomes. We also performed Trial Sequential Analyses (TSA).
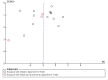
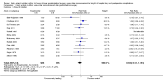
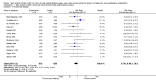
Main results: We identified 17 RCTs with 1437 participants undergoing lower gastrointestinal surgery. Most studies were at high or unclear risk of bias in two or more domains. Six studies were judged as having low risk of selection bias for random sequence generation and insufficient details were provided for judgement on allocation concealment in all 17 studies. With regards to performance and deception bias; 14 studies reported no attempt to blind participants and blinding of personnel was not discussed either. Only one study was judged as low risk of bias for blinding of outcome assessor. With regards to incomplete outcome data, three studies were judged to be at high risk because they had more than 10% difference in missing data between groups. For selective reporting, nine studies were judged as unclear as protocols were not provided and eight studies had issues with either missing data or incomplete reporting of results.LOS was reported in 16 studies (1346 participants). The mean LoS ranged from four days to 16 days in the early feeding groups and from 6.6 days to 23.5 days in the control groups. Mean difference (MD) in LoS was 1.95 (95% CI, -2.99 to -0.91, P < 0.001) days shorter in the early feeding group. However, there was substantial heterogeneity between included studies (I2 = 81, %, Chi2 = 78.98, P < 0.00001), thus the overall quality of evidence for LoS is low. These results were confirmed by the TSA showing that the cumulative Z-curve crossed the trial sequential monitoring boundary for benefit.We found no differences in the incidence of postoperative complications: wound infection (12 studies, 1181 participants, RR 0.99, 95%CI 0.64 to 1.52, very low-quality evidence), intraabdominal abscesses (6 studies, 554 participants, RR 1.00, 95%CI 0.26 to 3.80, low-quality evidence), anastomotic leakage/dehiscence (13 studies, 1232 participants, RR 0.78, 95%CI 0.38 to 1.61, low-quality evidence; number needed to treat for an additional beneficial outcome (NNTB) = 100), and pneumonia (10 studies, 954 participants, RR 0.88, 95%CI 0.32 to 2.42, low-quality evidence; NNTB = 333).Mortality was reported in 12 studies (1179 participants), and showed no between-group differences (RR = 0.56, 95%CI, 0.21 to 1.52, P = 0.26, I2 = 0%, Chi2 = 3.08, P = 0.96, low-quality evidence). The most commonly reported cause of death was anastomotic leakage, sepsis and acute myocardial infarction.Seven studies (613 participants) reported vomiting (RR 1.23, 95%CI, 0.96 to 1.58, P = 0.10, I2 = 0%, Chi2 = 4.98, P = 0.55, low-quality evidence; number needed to treat for an additional harmful outcome (NNTH) = 19), and two studies (118 participants) reported nausea (RR 0.95, 0.71 to 1.26, low-quality evidence). Four studies reported combined nausea and vomiting (RR 0.94, 95%CI 0.51 to 1.74, very low-quality evidence). One study reported QoL assessment; the scores did not differ between groups at 30 days after discharge on either QoL scale EORTC QLQ-C30 or EORTC QlQ-OV28 (very low-quality evidence).
Authors' conclusions: This review suggests that early enteral feeding may lead to a reduced postoperative LoS, however cautious interpretation must be taken due to substantial heterogeneity and low-quality evidence. For all other outcomes (postoperative complications, mortality, adverse events, and QoL) the findings are inconclusive, and further trials are justified to enhance the understanding of early feeding for these. In this updated review, only a few additional studies have been included, and these were small and of poor quality.To improve the evidence, future trials should address quality issues and focus on clearly defining and measuring postoperative complications to allow for better comparison between studies. However due to the introduction of fast track protocols which already include an early feeding component, future trials may be challenging. A more feasible trial may be to investigate the effect of differing postoperative energy intake regimens on relevant outcomes.
Conflict of interest statement
All authors: none known
Figures



















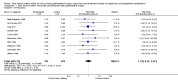
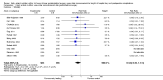



Update of
-
Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications.Cochrane Database Syst Rev. 2006 Oct 18;(4):CD004080. doi: 10.1002/14651858.CD004080.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2018 Oct 24;10:CD004080. doi: 10.1002/14651858.CD004080.pub3. PMID: 17054196 Updated.
References
References to studies included in this review
-
- Beier‐Holgersen R, Boesby S. Effect of early postoperative enteral nutrition on postoperative infections. [Danish]. Ugeskrift for Laeger 1998;160(22):3223‐6. - PubMed
- Beier‐Holgersen R, Boesby S. Influence of postoperative enteral nutrition on postsurgical infections. Gut 1996;39(6):833‐5. - PMC - PubMed
- Beier‐Holgersen R, Brandstrup B. Influence of early postoperative enteral nutrition versus placebo on cell‐mediated immunity, as measured with the Multitest CMI. Scandinavian Journal of Gastroenterology 1999;34(1):98‐102. - PubMed
- Beier‐Holgersen R, Brandstrup B. Influence of postoperative enteral nutrition on cellular immunity. A random double‐blinded placebo controlled clinical trial. International Journal of Colorectal Disease 2012;27(4):513‐20. - PubMed
-
- Binderow SR, Cohen SM, Wexner SD, Nogueras JJ. Must early postoperative oral intake be limited to laparoscopy?. Diseases of the Colon & Rectum 1994;37(6):584‐9. - PubMed
- Binderow SR, Cohen SM, Wexner SD, Schmitt SL, Nogueras JJ, Jagelman DG. Must early postoperative oral intake be limited to laparoscopy? [Abstract]. International Journal of Colorectal Disease 1993;8:230.
-
- Carr CS, Boulos PB. Immediate postoperative enteral feeding following bowel resection. [Abstract]. International Journal of Colorectal Disease 1996;11:142.
- Carr CS, Ling KD, Boulos P, Singer M. Randomised trial of safety and efficacy of immediate postoperative enteral feeding in patients undergoing gastrointestinal resection. BMJ 1996;312(7035):869‐71. - PMC - PubMed
- Singer M, Carr C. Immediate enteral feeding after gastrointestinal resection [Letter; author's reply]. BMJ 1996;313:230.
-
- Chatterjee S, Bala SK, Chakrabory P, Dey R, Sinha S, Ray R, et al. A comparative study between early enteral feeding (within 24 hours) versus conventional enteral feeding after enteric anastomosis. Bangladesh Journal of Medical Science 2012;11(4):273‐83.
-
- Consoli MLD, Fonseca LM, Silva RG, Davisson Correia MIT. Early postoperative oral feeding impacts positively in patients undergoing colonic resection: results of a pilot study. Nutrición Hospitalaria 2010;25(5):806‐9. - PubMed
- Fonseca LM, Profeta da Luz MM, Lacerda‐Filho A, Davisson Correia MIT, Silva RG. A simplified rehabilitation program for patients undergoing elective colonic surgery ‐ randomized controlled clinical trial. International Journal of Colorectal Disease 2011;26(5):609‐16. - PubMed
References to studies excluded from this review
-
- Barletta JF, Asgeirsson T, Senagore AJ. Influence of intravenous opioid dose on postoperative ileus. Annals of Pharmacotherapy 2011;45(7‐8):916‐23. - PubMed
-
- Barlow R, Price P, Reid TD, Hunt S, Clark GW, Havard TJ, et al. Prospective multicentre randomised controlled trial of early enteral nutrition for patients undergoing major upper gastrointestinal surgical resection. Clinical Nutrition 2011;30(5):560‐6. - PubMed
-
- Beati C, Samori G, Gentile MG, Corradi E, Gastaldo L, Confalonieri MA. A prospective, randomised trial of early enteral feeding after resection for colorectal cancer. British Journal of Surgery 1998;85:69.
References to studies awaiting assessment
-
- Fanaie SA, Ziaee SA. Safety of early oral feeding after gastrointestinal anastomosis: a randomized controlled trial. Indian Journal of Surgery 2005;67(4):185‐8.
Additional references
-
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. Journal of National Cancer Institute 1993;85:365–7. - PubMed
-
- Ahmed J, Khan S, Lim M, Chandrasekaran TV, MacFie J. Enhanced recovery after surgery protocols – compliance and variations in practice during routine colorectal surgery. Colorectal Disease 2012;14(9):1045‐51. - PubMed
-
- Bradburn MJ, Deeks JJ, Berlin JA, Localio RA. Much ado about nothing: A comparison of the performance of meta‐analytical methods with rare events. Statistics in Medicine 2007;26(1):53‐77. - PubMed
-
- Buzby GP, Knox LS, Crosby LO, Eisenberg JM, Haakenson CM, McNeal GE, et al. Study protocol: a randomized clinical trial of total parenteral nutrition in malnourished surgical patients.. Amercian Juornal of Clinical Nutrition 1988;47:366‐81. - PubMed
-
- Cortes J, Gonzalez JA, Campbell MJ, Cobo E. A hazard ratio was estimated by a ratio of median survival times, but with considerable uncertainty. Journal of Clinical Epidemiology 2014;67(10):1172‐7. - PubMed
References to other published versions of this review
-
- Andersen HK, Lewis SJ, Thomas S. Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Database of Systematic Reviews 2011;18(4):CD004080. - PubMed
-
- Lewis SJ, Andersen HK, Thomas S. Early enteral nutrition within 24 h of intestinal surgery versus later commencement of feeding: a systematic review and meta‐analysis. Journal of Gastrointestinal Surgery 2009;13(3):569‐75. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

